GenSight Biologics reports positive follow-up results at Week 72 of the RESCUE Phase III clinical trial of GS010 in Leber Hereditary Optic Neuropathy (LHON)
- GS010-treated eyes showed continuous recovery of visual functions from nadir (worst vision post-treatment) in both best-corrected visual acuity (BCVA) and contrast sensitivity (CS)
- GS010-treated eyes recovered 21 ETDRS letters from nadir at Week 72
- Bilateral improvements in visual function corroborate previously observed parallel evolution of GS010- and sham-treated eyes in both RESCUE and REVERSE trials
- Mean BCVA in both GS010- and sham-treated eyes improved from off-chart values at Week 48 to on-chart values at Week 72
- 40% of eyes achieved a clinically meaningful BCVA improvement from nadir (0.3 LogMAR or 15 ETDRS letters) at Week 72
- GS010 continued to be safe and well-tolerated through 72 weeks
Paris, France, April 17, 2019, 7:30 a.m. CET – GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today announced results from the second scheduled readout, at Week 72, of the RESCUE Phase III clinical trial evaluating the safety and efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in 39 subjects whose visual loss due to 11778-ND4 Leber Hereditary Optic Neuropathy (LHON) occurred up to 6 months prior to study treatment. These subjects received GS010 in one eye and a sham injection in the other eye, with drug treatment randomized between best- and worst-affected eyes.
The key measure of visual function – best-corrected visual acuity (BCVA) – continued to improve at Week 72 compared to Week 48, demonstrating sustained recovery from the lowest point, or nadir, experienced in the acute phase of the disease. By Week 72, GS010-treated eyes improved by -0.413 LogMAR (+21 ETDRS letters equivalent) from nadir, compared to the Week 48 improvement of -0.257 LogMAR (+13 ETDRS letters equivalent). This recovery at week 72 could not yet completely offset deterioration from baseline through the acute phase: GS010-treated eyes were still below baseline by 0.192 LogMAR (-10 ETDRS letters equivalent), compared to 0.380 LogMAR (-19 ETDRS letters equivalent) at Week 48.
Figure 1. Time Course Visual Acuity, Change from Baseline to Week 72 in ETDRS Letters Equivalent in RESCUE
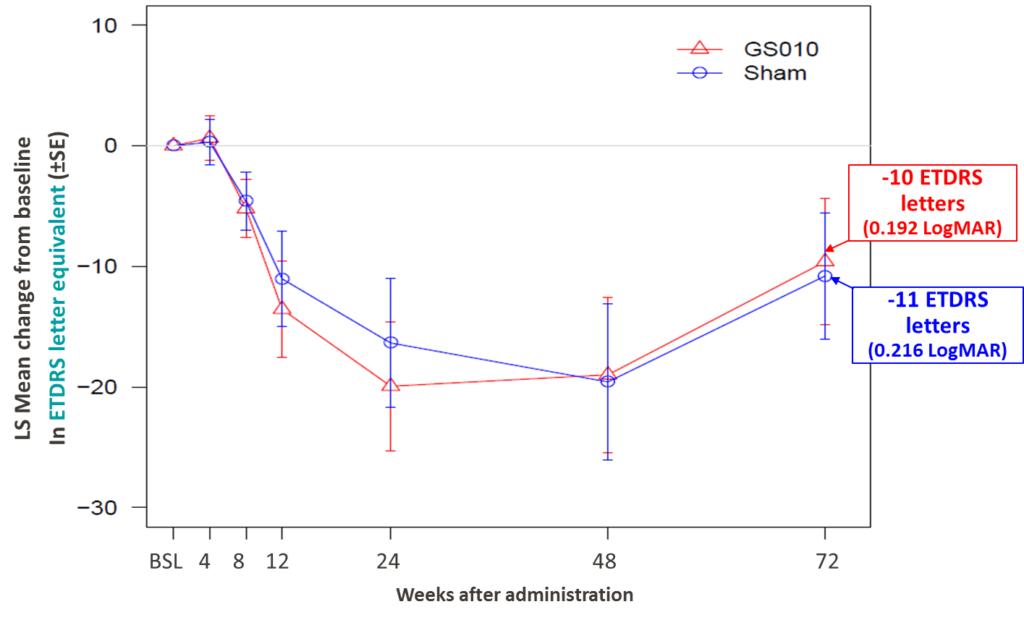
Consistent with all readouts so far in the RESCUE and REVERSE trials, sham-treated eyes had a BCVA evolution that closely tracked that of GS010-treated eyes. At Week 72 of RESCUE, sham-treated eyes improved by -0.435 LogMAR from nadir (+21.7 ETDRS letters equivalent). The U-shaped curve thus closely matched that of GS010-treated eyes, so a statistically significant difference in visual acuity between GS010- and sham-treated eyes could not be shown.
The strength of the bilateral recovery shifted the mean BCVA in both sets of eyes from being off-chart at Week 48 to on-chart at Week 72. In addition, 40% of GS010- and sham-treated eyes improved by a clinically meaningful difference of -0.3 LogMAR (+15 letters ETDRS) from nadir. Similarly, 58% of GS010-treated and 50% of sham-treated eyes improved by a clinically meaningful difference of -0.2 LogMAR (+10 lett ETDRS) from nadir.
Figure 2. Time Course LogMAR Visual Acuity to 72 Weeks in RESCUE
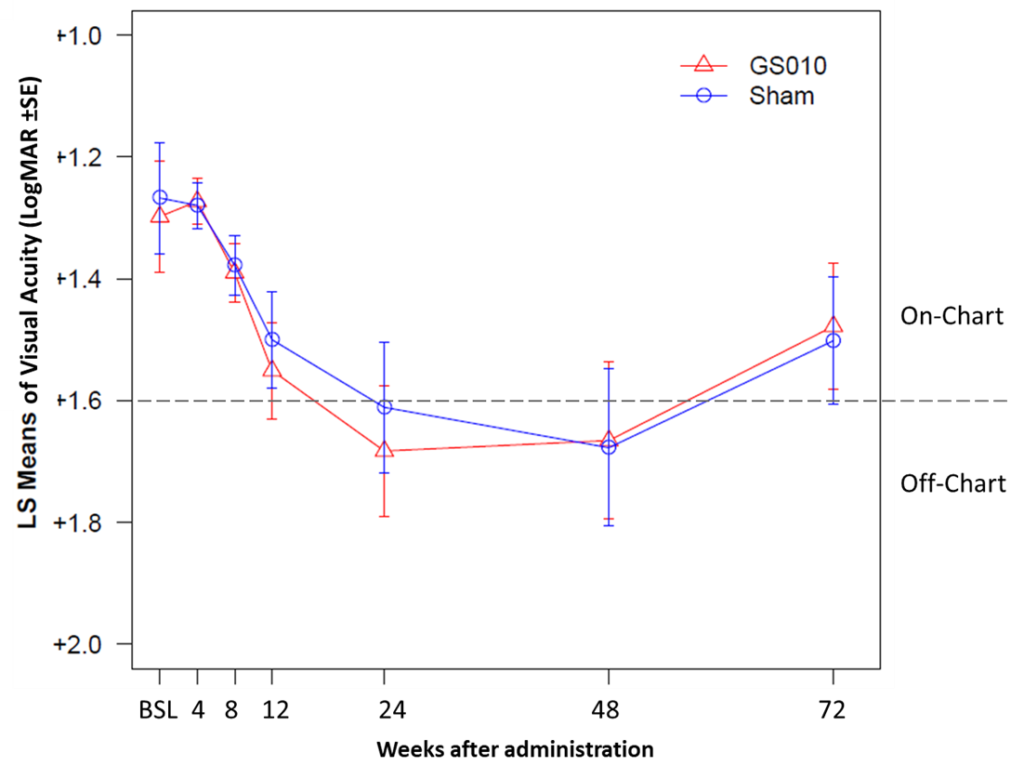
Note: LS Means = Least Squares Means
Table 1. Change from Nadir* in Best-Corrected Visual Acuity
(LogMAR and ETDRS Letter Equivalents)
| LogMAR Visual Acuity | ETDRS Letter Equivalent | |||||
| Week 48 | Week 72 | Week 48 | Week 72 | |||
| n | Mean (SD) | n | Mean (SD) | |||
| GS010-Treated eyes | 36 | -0.257(0.358) | -0.413(0.527) | 34 | +12.8(17.9) | +20.6(26.3) |
| Sham-Treated Eyes | 36 | -0.236(0.319) | -0.435(0.501) | 33 | +11.8(15.6) | +21.7(25.1) |
Note: *As per Statistical Analysis Plan (SAP), nadir was defined as the lowest post-treatment LogMAR value up to week of interest. Light Perception/No Light Perception, or LP/NLP, vision was not included in the analysis.
Contrast sensitivity (CS), a second visual function, evolved in a manner similar to BCVA: while values for GS010-treated eyes and sham-treated eyes remained below baseline, CS also recovered so that the gap to baseline diminished at Week 72 compared to Week 48. The two sets of eyes closely matched each other, so that the difference between their CS values was not statistically significant.
Figure 3. Time Course LogCS Contrast Sensitivity to 72 Weeks in RESCUE
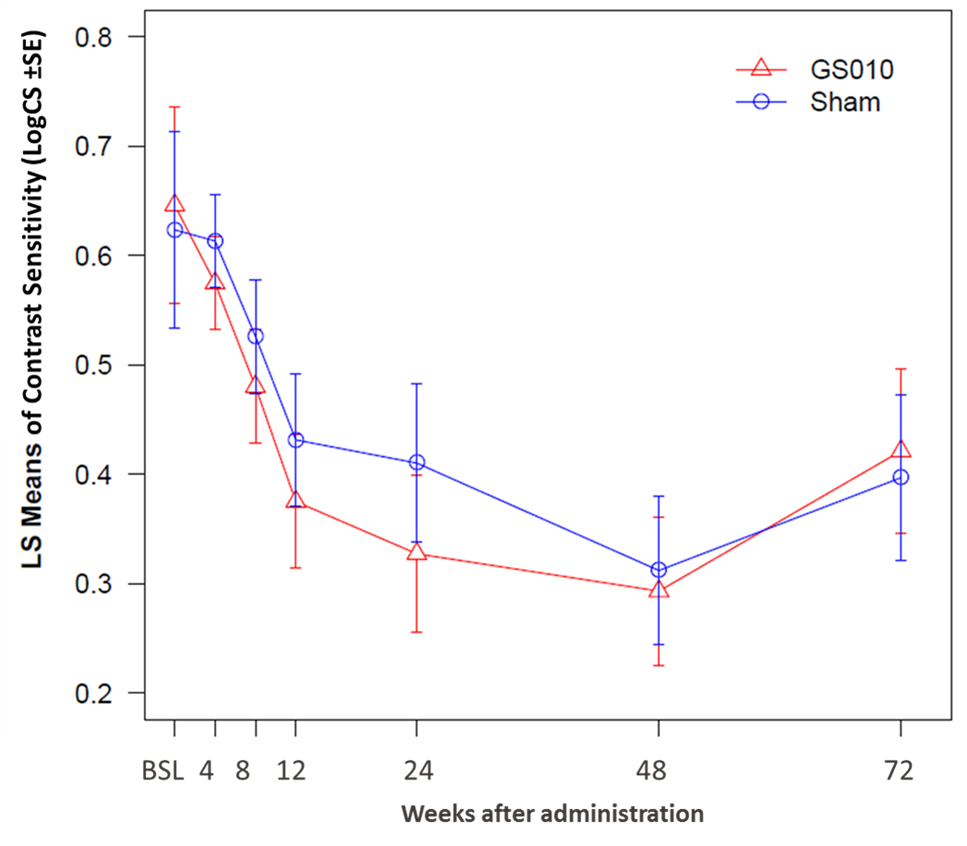
Note: LS Means = Least Squares Means
Table 2. Change of Contrast Sensitivity from Baseline
(LogCS)
| Week 48 | Week 72 | |||
| n | Least-Squares Mean (Standard Error) | n | Least-Squares Mean (Standard Error) | |
| All GS010-Treated eyes | 36 | -0.34(0.07) | 38 | -0.25(0.07) |
| All Sham-Treated Eyes | 36 | -0.32(0.07) | 38 | -0.28(0.07) |
Note: A mixed model of analysis of covariance (ANCOVA) was used with change from baseline at week 72 as the response.
“This improvement in visual function from Week 48 to Week 72, both in visual acuity and contrast sensitivity, strengthens our belief in the benefits of GS010, looking at the shift of the mean BCVA from off-chart to on-chart at Week 72. These results show a more favorable trend than the outcome we usually observe in clinical practice for LHON ND4 patients,” commented Dr. Catherine Vignal, Head of the department of Neuro-Ophthalmology at the Rothschild Foundation, and Principal Investigator at the Department of Ophthalmology at Centre Hospitalier National d’Ophtalmologie des XV-XX, Paris.
One difference between results from the REVERSE and RESCUE trials of GS010 lies in anatomic findings. The data so far do not indicate differential protection for the anatomy of GS010-treated eyes: in both drug-treated and sham-treated eyes, the relevant anatomy, as shown by various OCT measurements (tRNFL thickness, PMB thickness, GCL volume), continued to thin at Week 72, although the rate of thinning decreased between Week 48 and Week 72. Among the OCT measures in the trial at Week 72, the ETDRS macular volume showed a difference between GS010-treated and sham-treated eyes (0.096 mm3, p = 0.0012).
“By design, the population in Rescue is very heterogeneous, and the structure of their retina is also highly variable, from marked atrophy of nerve fibers to edema. At unmasking, which happens after week 96, we will separate our subjects by their baseline OCT findings. In the sub-group with edema, thinning over time will be a good finding. In those with baseline atrophy of nerve fibers, increases in thickness will be a good sign. This mix of OCT findings at entry masks the true OCT findings at week 72 in Rescue,” commented Dr. Robert C. Sergott, Director, Wills Eye Hospital, Neuro-Ophthalmology and Director, William H. Annesley, Jr, EyeBrain Center, Thomas Jefferson University, Philadelphia, PA.
Based on preliminary analysis of the safety data, GS010 was well-tolerated through 72 weeks. There were no serious ocular adverse events or discontinuations due to ocular issues. The most frequently seen ocular adverse events were related to the injection procedure itself. Transient elevations of intraocular pressure were occasionally seen but secondary to intraocular inflammation likely due to administration of GS010. Such episodes were without sequelae and responded to conventional treatment. There were no systemic serious adverse events or discontinuations related to study treatment or study procedure.
“We are excited and extremely gratified to see a picture of a sustained recovery of visual function emerge from RESCUE results at Week 72,” said Bernard Gilly, Co-founder and Chief Executive Officer of GenSight. “The findings continue to be consistent with what was observed in REVERSE at 48 and 72 weeks as well as with RESCUE at 48 weeks, and bolster our confidence in the benefits that GS010 can deliver to patients and motivate us to work with the authorities to bring GS010 as early as possible to the market.”
RESCUE subjects will be evaluated again at 96 weeks, and then data will be unmasked, allowing more detailed subject-level analyses to be conducted. Results from RESCUE at Week 96 are expected to be available by the end of Q3 2019. Week 96 data from the REVERSE trial are expected earlier, in May 2019.
The third interventional study for GS010, REFLECT, is a randomized, double-masked, placebo-controlled Phase III trial evaluating the safety and efficacy of bilateral injections of GS010 in patients up to one year from onset of vision loss due to LHON. The first patient in REFLECT was treated in March 2018.
The Company will host a conference call today, April 17, 2019, at 10am CEST in French, and at 2.30pm CEST (8.30am EST) in English, to discuss these results.
Webcast & Conference call in French
Dial-in numbers:
- France: +33 (0) 1 7037 7166
- United Kingdom: +44 (0) 20 3003 2666
- Password: GenSight
Webcast link: https://channel.royalcast.com/webcast/gensightbiologicsfr/20190417_1/
Webcast & Conference call in English
Dial-in numbers:
- United States: +1 212 999 6659
- France: +33 (0) 1 7037 7166
- United Kingdom: +44 (0) 20 3003 2666
- Password: GenSight
Webcast link: https://channel.royalcast.com/webcast/gensightbiologicsen/20190417_1/
A replay of the calls and webcasts will be available by using the above links.
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to affected eyes by intravitreal injection to offer patients a sustainable functional visual recovery.
About GS010
GS010 targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of patients have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously. The estimated incidence of LHON is approximately 1,400 to 1,500 new patients who lose their sight every year in the United States and Europe.
About RESCUE and REVERSE
RESCUE and REVERSE are two separate randomized, double-masked, sham-controlled Phase III trials designed to evaluate the efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in subjects affected by LHON due to the G11778A mutation in the mitochondrial ND4 gene.
The primary endpoint will measure the difference in efficacy of GS010 in treated eyes compared to sham-treated eyes based on Best‑Corrected Visual Acuity (BCVA), as measured with the ETDRS at 48 weeks post-injection. The patients’ LogMAR (Logarithm of the Minimal Angle of Resolution) scores, which are derived from the number of letters patients read on the ETDRS chart, will be used for statistical purposes. Both trials have been adequately powered to evaluate a clinically relevant difference of at least 15 ETDRS letters between treated and untreated eyes adjusted to baseline.
The secondary endpoints will involve the application of the primary analysis to best‑seeing eyes that received GS010 compared to those receiving sham, and to worse‑seeing eyes that received GS010 compared to those that received sham. Additionally, a categorical evaluation with a responder analysis will be evaluated, including the proportion of patients who maintain vision (< ETDRS 15L loss), the proportion of patients who gain 15 ETDRS letters from baseline and the proportion of patients with Snellen acuity of >20/200. Complementary vision metrics will include automated visual fields, optical coherence tomography, and color and contrast sensitivity, in addition to quality of life scales, bio‑dissemination and the time course of immune response. By protocol, readouts for these endpoints are at 48, 72 and 96 weeks after injection.
The trials are conducted in parallel, with 37 subjects for REVERSE and 39 subjects for RESCUE, in 7 centers across the United States, the UK, France, Germany and Italy. Week 96 results are expected in 2019 for both trials, after which patients will be transferred to a long-term follow-up study that will last for three years.
ClinicalTrials.gov Identifiers:
REVERSE: NCT02652780
RESCUE: NCT02652767
About REFLECT
REFLECT is a multi-center, randomized, double-masked, placebo-controlled study to evaluate the safety and efficacy of bilateral injections of GS010 in subjects with LHON due to the NADH dehydrogenase 4 (ND4) mutation.
The trial is planned to enroll 90 patients with vision loss up to 1 year in duration and will be conducted in multiple centers in Europe and in the US.
In the active arm, GS010 will be administered as a single intravitreal injection to both eyes of each subject. In the placebo arm, GS010 will be administered as a single intravitreal injection to the first affected eye, while the fellow eye will receive a placebo injection.
The primary endpoint for the REFLECT trial is the BCVA reported in LogMAR at 1-Year post-treatment in the second‑affected/not‑yet‑affected eye. The change from baseline in second‑affected/not‑yet‑affected eyes receiving GS010 and placebo will be the primary response of interest. The secondary efficacy endpoints include: BCVA reported in LogMAR at 2-Years post-treatment in the second‑affected/not‑yet‑affected eye compared to both placebo and the first‑affected eye receiving GS010, OCT, color and contrast sensitivity and quality of life scales. The first subject was treated in March 2018.
ClinicalTrials.gov Identifiers:
REFLECT: NCT03293524
CONTACTS
-
GENSIGHT BIOLOGICSChief Financial OfficerThomas Gidoin+33 (0)1 76 21 72 20
-
Source: GenSight Biologics


