GenSight Biologics reports positive 96-week data from REVERSE Phase III clinical trial of GS010 for the treatment of Leber Hereditary Optic Neuropathy (LHON)
- Durable improvement in best-corrected visual acuity (BCVA), as GS010-treated eyes sustain +15.4 ETDRS letters equivalent improvement versus baseline and +28.1 ETDRS letters equivalent versus nadir
- Bilateral improvement continues in key visual functions BCVA and contrast sensitivity
- Responder analyses suggest improved clinical outcomes for GS010-treated eyes relative to sham-treated eyes
- GS010 safety and tolerability established over the course of the trial
- Briefing packages incorporating findings under preparation for meetings with regulatory authorities
Paris, France, Wednesday, May 15, 2019, 7.30 am CEST – GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today reported a first set of results from Week 96 of the REVERSE Phase III clinical trial. The trial evaluated the safety and efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in 37 subjects whose visual loss due to 11778-ND4 Leber Hereditary Optic Neuropathy (LHON) commenced between 6 and 12 months prior to study treatment. Week 96 is the last of the scheduled readouts for the trial and marks the time when the data are unmasked, providing access to individual patient profiles.
The results point to continued efficacy of GS010 two years past injection, with best-corrected visual acuity (BCVA) sustaining a clinically meaningful improvement over baseline. At Week 96, GS010-treated eyes showed a mean improvement of -0.308 LogMAR compared to baseline, equivalent to +15.4 ETDRS letters or 3 lines on the ETDRS vision chart. This clinically meaningful level of improvement in visual acuity maintains the gain observed at Week 72 (+14.7 ETDRS letters equivalent).
As in previous readouts at Week 48 and Week 72, BCVA in sham-treated eyes evolved on a relatively parallel trajectory, achieving a mean improvement of -0.259 LogMAR over baseline, or a gain of +12.9 ETDRS letters equivalent, at Week 96. Although lower in magnitude, the mean BCVA improvement of sham-treated eyes was not statistically significant from that of GS010-treated eyes.
Figure 1: Sustained bilateral improvement in BCVA in REVERSE
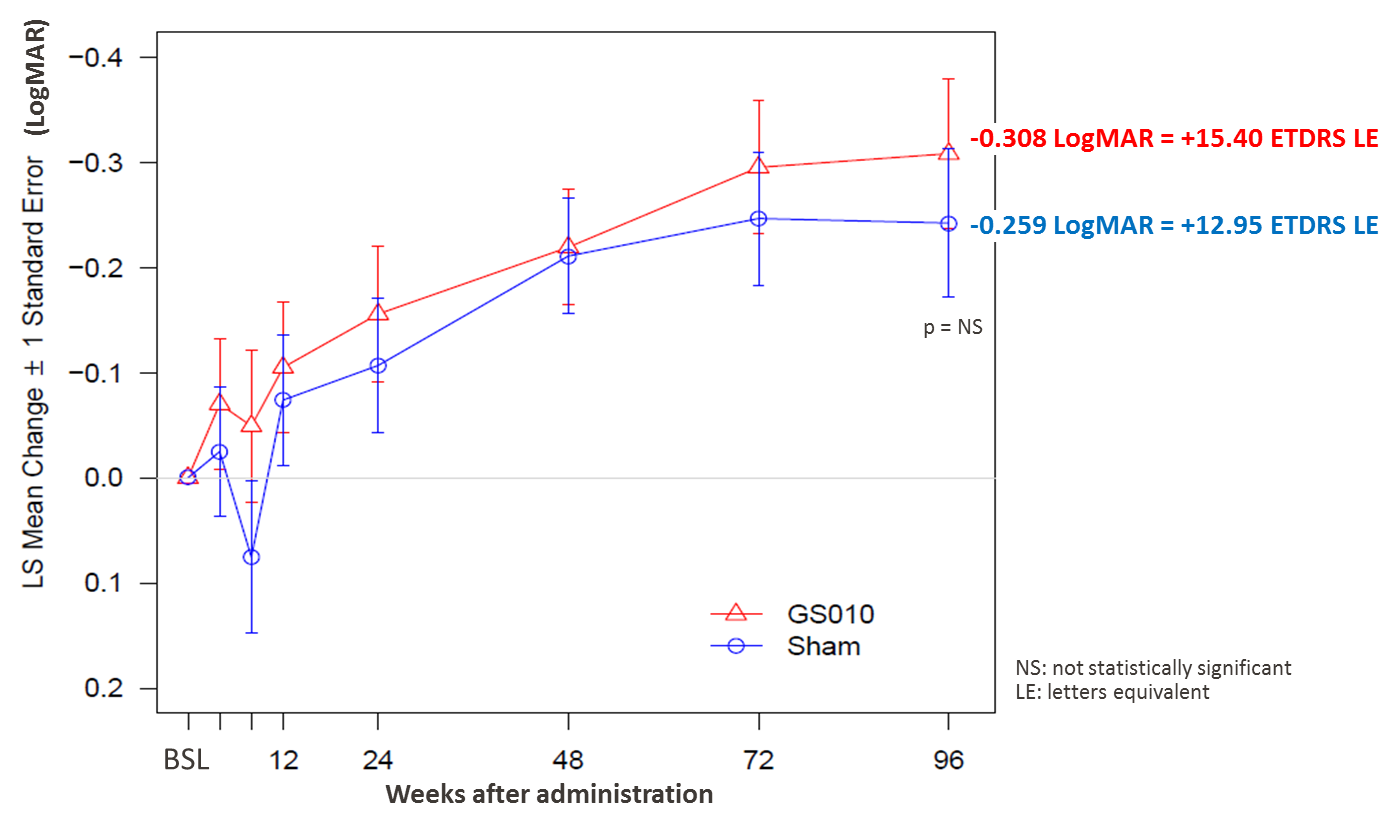
Notes: A mixed model of analysis of covariance (ANCOVA) was used with change from baseline as the response, and subject, eyes of the subject as random factor, treatment and the baseline LogMAR value as covariates in the model.
As in RESCUE and consistent with natural history, subjects experienced an initial point of low visual acuity, or nadir. Eyes of REVERSE subjects recovered impressively. By week 96, GS010-treated eyes had gained +28 more letters relative to their nadir.
Table 1. Recovery of BCVA from Nadir*: RESCUE and REVERSE
(Difference** from Nadir* in ETDRS Letters Equivalent, Mean and Standard Deviation)
| RESCUE | REVERSE | |||||||
| n | Week 48 | n | Week 72 | n | Week 48 | Week 72 | Week 96 | |
| GS010-treated eyes | 36 | +12.9(17.9) | 34 | +20.6(26.4) | 37 | +24.1(21.1) | +27.4(21.8) | +28.1(22.0) |
| Sham-treated eyes | 36 | +11.8(16.0) | 33 | +21.8(25.1) | 37 | +20.3(23.4) | +22.6(25.5) | +23.2(24.5) |
Note: *Nadir was defined as the lowest post-treatment BCVA as measured by LogMAR up to week of interest. Light Perception/No Light Perception, or LP/NLP, vision was not included in the analysis. **Change from nadir was first calculated using observed LogMAR values; no data were imputed. The LogMAR values were then converted to the corresponding ETDRS letters equivalent.
At Week 96, a second key visual function – low-contrast visual acuity, as measured on the Pelli-Robson chart – showed a similar trend of improvement for both GS010-treated eyes and sham-treated eyes.
Figure 2: Evolution of contrast sensitivity in REVERSE
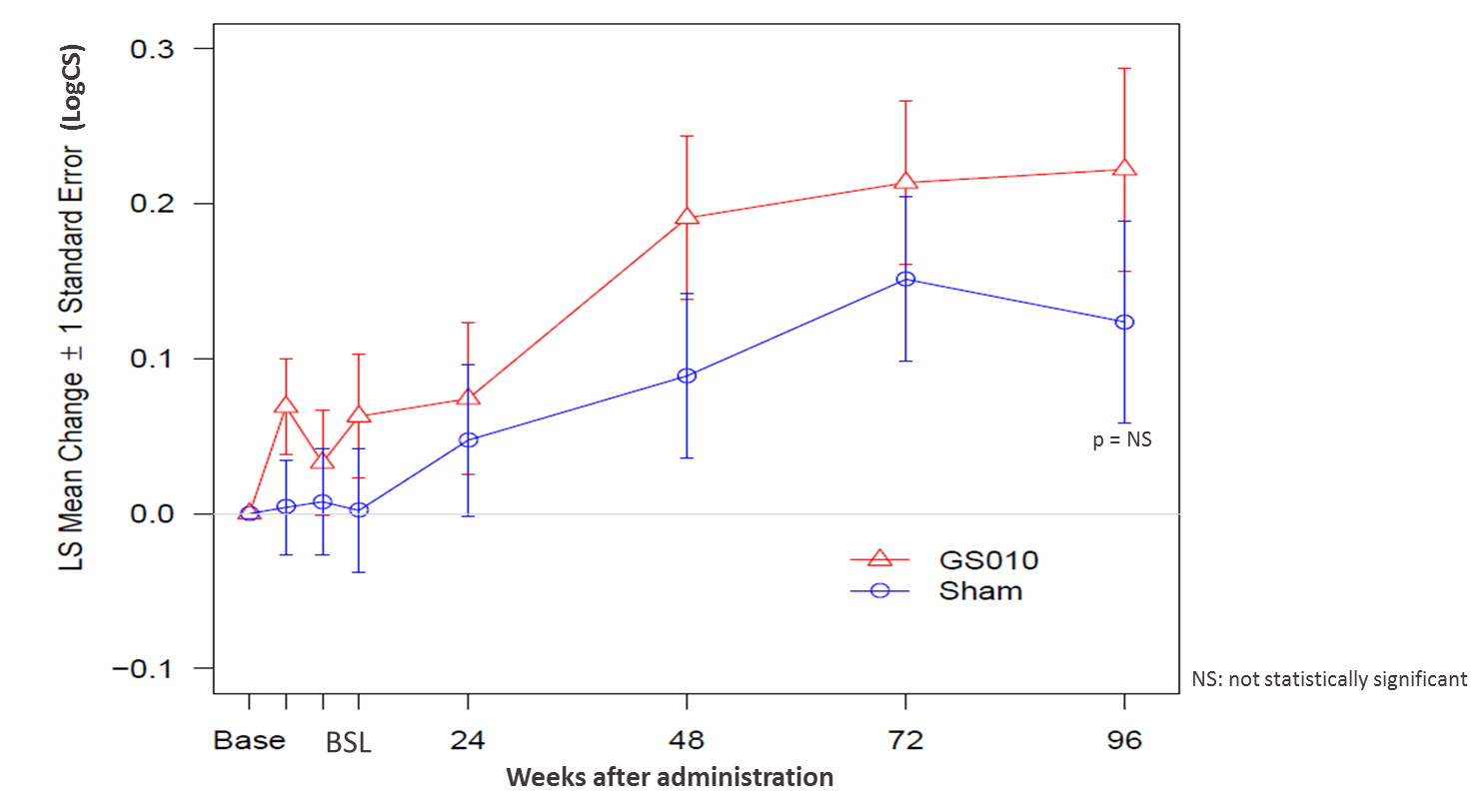
Note: An analysis of covariance (ANCOVA), with repeated measures for eye and covariate adjustment for baseline LogCS, was used to model the effect of treatment on change in LogCS from baseline. A separate analysis was performed at each time point.
The trajectories, however, did not track each other as closely as with BCVA: mean contrast sensitivity for GS010-treated eyes show a more robust improvement versus baseline over the course of the trial. Nonetheless, the drop in the mean contrast sensitivity of sham-treated eyes was not enough to yield a statistically significant difference in Week 96 mean contrast sensitivity improvement between the two sets of eyes.
“The data show that both the treated and the sham eye improved in both high and low contrast, defying the accepted natural history of this disease and improving upon it, based upon the clinical experiences of generations of neuro-ophthalmologists,” commented Dr. Robert C. Sergott, Director, Wills Eye Hospital, Neuro-Ophthalmology and Director, William H. Annesley, Jr, EyeBrain Center, Thomas Jefferson University, Philadelphia, PA. “The behavior of the untreated eye must also make us re-examine what we thought we knew as possibly dogma and be open to the idea that gene therapy delivered into one eye may be able to access the contralateral eye.”
“It is encouraging that GS010-treated eyes were nearly four times more likely to achieve vision better than 20/200 compared with sham eyes. The next step, which is to analyze individual longitudinal data on the visual parameters for each subject recruited into REVERSE, should further clarify the therapeutic benefit of GS010 in 11778-ND4 LHON,” noted Dr. Patrick Yu-Wai Man, Senior Lecturer and Honorary Consultant Ophthalmologist at the University of Cambridge, Moorfields Eye Hospital, and the UCL Institute of Ophthalmology, London, United Kingdom.
Another responder analysis provides a useful perspective on the REVERSE results. In a natural history study conducted by Santhera1, 15% of subjects who had the 11778A mutation achieved the following definition of spontaneous “clinically relevant recovery” (CRR) from baseline in at least one eye:
- Improved by at least 10 ETDRS letters from on-chart visual acuity, or
- Improved from off-chart visual acuity to being able to read at least 5 ETDRS letters
By comparison, 68% of REVERSE subjects achieved this definition of CRR in at least one eye at Week 96, with GS010-treated eyes significantly more likely to achieve this than sham-treated eyes (62% vs. 43%, p = 0.0348).
Improvements in visual function were reflected in quality of life scores in the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25) survey, a validated, vision-specific quality-of-life instrument completed by REVERSE subjects. Mean composite score and means of relevant sub-scale scores continued to improve over baseline, particularly for the ability to carry out near and distance activities. The increase over baseline of the mean sub-scale scores exceeded those that have been associated with a 15-letter improvement in BCVA in other ocular diseases.
Table 2: Meaningful improvements in Quality of Life Scores Reported by REVERSE Subjects
NEI VFQ-25 Results from REVERSE
Mean change from baseline (absolute score and percent)
| Composite Score** | Near Activities | Distance Activities | Dependency | Role Difficulties | General Vision | Mental Health | |
| Week 48 | +7.2+23.2% | +10.4+65.1% | +9.6+49.8% | +12.4+100.6% | +14.5+65.0% | +10.3+50.9% | +11.2+81.9% |
| Week 72 | +8.1+25.2% | +9.5+58.1% | +8.2+42.5% | +18.9+130.2% | +15.2+70.9% | +11.9+54.1% | +15.2+105.6% |
| Week 96 | +9.5+28.8% | +13.3+78.1% | +10.7+47.4% | +18.5130.2% | +15.9+78.9% | +6.5+32.4% | +16.1+108.2% |
| Clinically relevant difference* | +3.90 to +4.34 | +4.67 to +6.06 | +5.15 to +5.38 | +4.72 to +4.98 | +3.31 to +4.70 | +4.38 to +4.82 | +4.70 to +4.88 |
*Suñer et al. (2009): clinically relevant score differences based on a clinically significant 15-letter BCVA improvement at 12 months.
**The composite score is an average of the vision-targeted sub-scale scores, excluding the general health rating question.
Note: The mean percent improvement for a sub-score was calculated by averaging the individual percent improvements.
Structural metrics indicate that GS010-treated eyes maintained the stability achieved in previous readouts in ganglion cell volume. The differential effect of therapy was, however, more prominent in previous readouts.
Initial analyses of subject-level data suggest that the bilateral effect is common across patients; other analyses of subject-level data are ongoing. GenSight will hold an investor meeting (in English, to be simulcast in French) on May 23 in New York City, where invited key opinion leaders will discuss results in more detail.
Week 96 data further establish the favorable safety profile of GS010. There have been no discontinuations in the trial and no serious adverse effects in GS010-treated eyes. The ocular AEs most frequently reported in the therapy group were mainly related to the injection procedure itself, along with the occurrence of intraocular inflammation (accompanied by elevation of intraocular pressure in some patients), likely related to GS010; these were responsive to conventional treatment and were without sequelae.
“We are very gratified by these remarkable findings, which demonstrate the durable and clinically meaningful impact of GS010, while continuing to establish the safety of our gene therapy,” commented Bernard Gilly, Co-founder and Chief Executive Officer of GenSight. “We are moving with speed and determination to build a compelling dossier from the results of REVERSE and, later this year, RESCUE.”
REVERSE Week 96 results will be included in the briefing materials that are being prepared for a pre-submission meeting with the EMA and a Type B meeting with the FDA, both of which are planned for mid-year.
Data from the 96-week readout of the second Phase III trial for GS010, RESCUE, is expected to be available by the end of Q3 2019.
The third interventional study for GS010, REFLECT, is a randomized, double-masked, placebo-controlled Phase III trial evaluating the safety and efficacy of bilateral injections of GS010 in patients up to one year from onset of vision loss due to LHON. The first patient in REFLECT was treated in March 2018; treatment of the last patient is expected in Q2 of this year.
GenSight will host a conference call today, May 15, 2019, at 10am CEST in French, and at 2.30pm CEST (8.30am EST) in English, to discuss these results.
Webcast & Conference call in French
Dial-in numbers:
- United States: +1 212 999 6659
- France: +33 (0) 1 7037 7166
- United Kingdom: +44 (0) 20 3003 2666
- Password: GenSight
Webcast link: https://channel.royalcast.com/webcast/gensightbiologicsfr/20190514_1/
Webcast & Conference call in English
Dial-in numbers:
- United States: +1 212 999 6659
- France: +33 (0) 1 7037 7166
- United Kingdom: +44 (0) 20 3003 2666
- Password: GenSight
Webcast link: https://channel.royalcast.com/webcast/gensightbiologicsen/20190514_1/
A replay of the calls and webcasts will be available by using the above links.
Reference:
- Magda et al (2019), “Natural History of Leber’s Hereditary Optic Neuropathy (LHON): Findings from a Large Patient Cohort”, Poster presented at NANOS March 16-21, 2019; Poster Session II: Scientific Advancements; Poster: 163
About GenSight Biologics
GenSight Biologics S.A. is a clinical-stage biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders. GenSight Biologics’ pipeline leverages two core technology platforms, the Mitochondrial Targeting Sequence (MTS) and optogenetics, to help preserve or restore vision in patients suffering from blinding retinal diseases. GenSight Biologics’ lead product candidate, GS010, is in Phase III trials in Leber Hereditary Optic Neuropathy (LHON), a rare mitochondrial disease that leads to irreversible blindness in teens and young adults. Using its gene therapy-based approach, GenSight Biologics’ product candidates are designed to be administered in a single treatment to each eye by intravitreal injection to offer patients a sustainable functional visual recovery.
About GS010
GS010 targets Leber Hereditary Optic Neuropathy (LHON) by leveraging a mitochondrial targeting sequence (MTS) proprietary technology platform, arising from research conducted at the Institut de la Vision in Paris, which, when associated with the gene of interest, allows the platform to specifically address defects inside the mitochondria using an AAV vector (Adeno-Associated Virus). The gene of interest is transferred into the cell to be expressed and produces the functional protein, which will then be shuttled to the mitochondria through specific nucleotidic sequences in order to restore the missing or deficient mitochondrial function.
About Leber Hereditary Optic Neuropathy (LHON)
Leber Hereditary Optic Neuropathy (LHON) is a rare maternally inherited mitochondrial genetic disease, characterized by the degeneration of retinal ganglion cells that results in brutal and irreversible vision loss that can lead to legal blindness, and mainly affects adolescents and young adults. LHON is associated with painless, sudden loss of central vision in the 1st eye, with the 2nd eye sequentially impaired. It is a symmetric disease with poor functional visual recovery. 97% of patients have bilateral involvement at less than one year of onset of vision loss, and in 25% of cases, vision loss occurs in both eyes simultaneously. The estimated incidence of LHON is approximately 1,400 to 1,500 new patients who lose their sight every year in the United States and Europe.
About RESCUE and REVERSE
RESCUE and REVERSE are two separate randomized, double-masked, sham-controlled Phase III trials designed to evaluate the efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in subjects affected by LHON due to the G11778A mutation in the mitochondrial ND4 gene.
The primary endpoint will measure the difference in efficacy of GS010 in treated eyes compared to sham-treated eyes based on Best‑Corrected Visual Acuity (BCVA), as measured with the ETDRS at 48 weeks post-injection. The patients’ LogMAR (Logarithm of the Minimal Angle of Resolution) scores, which are derived from the number of letters patients read on the ETDRS chart, will be used for statistical purposes. Both trials have been adequately powered to evaluate a clinically relevant difference of at least 15 ETDRS letters between treated and untreated eyes adjusted to baseline.
The secondary endpoints will involve the application of the primary analysis to best‑seeing eyes that received GS010 compared to those receiving sham, and to worse‑seeing eyes that received GS010 compared to those that received sham. Additionally, a categorical evaluation with a responder analysis will be evaluated, including the proportion of patients who maintain vision (< ETDRS 15L loss), the proportion of patients who gain 15 ETDRS letters from baseline and the proportion of patients with Snellen acuity of >20/200. Complementary vision metrics will include automated visual fields, optical coherence tomography, and color and contrast sensitivity, in addition to quality of life scales, bio‑dissemination and the time course of immune response. Readouts for these endpoints are at 48, 72 and 96 weeks after injection.
The trials are conducted in parallel, in 37 subjects for REVERSE and 39 subjects for RESCUE, in 7 centers across the United States, the UK, France, Germany and Italy. Week 96 results are expected in 2019 for both trials, after which patients will be transferred to a long-term follow-up study that will last for three years.
ClinicalTrials.gov Identifiers:
REVERSE: NCT02652780
RESCUE: NCT02652767
About REFLECT
REFLECT is a multi-center, randomized, double-masked, placebo-controlled study to evaluate the safety and efficacy of bilateral injections of GS010 in subjects with LHON due to the NADH dehydrogenase 4 (ND4) mutation.
The trial is planned to enroll 90 patients with vision loss up to 1 year in duration and will be conducted in multiple centers in Europe and in the US.
In the active arm, GS010 will be administered as a single intravitreal injection to both eyes of each subject. In the placebo arm, GS010 will be administered as a single intravitreal injection to the first affected eye, while the fellow eye will receive a placebo injection.
The primary endpoint for the REFLECT trial is the BCVA reported in LogMAR at 1-Year post-treatment in the second‑affected/not‑yet‑affected eye. The change from baseline in second‑affected/not‑yet‑affected eyes receiving GS010 and placebo will be the primary response of interest. The secondary efficacy endpoints include: BCVA reported in LogMAR at 2-Years post-treatment in the second‑affected/not‑yet‑affected eye compared to both placebo and the first‑affected eye receiving GS010, OCT and contrast sensitivity and quality of life scales. The first subject was treated in March 2018.
ClinicalTrials.gov Identifiers:
REFLECT: NCT03293524
Source: GenSight Biologics


