Our Portfolio
HealthCap is a life science investor and company creator. Our motivation is to find innovative ideas and invest in people and companies that could influence healthcare and society. Together with our stakeholders, we strive to make a real impact and create a better world.
Portfolio Companies
Approved Products
All Companies
Current
Former

Country
Switzerland
Actelion Holding Ltd
Actelion is a Swiss biopharmaceutical company focusing on pulmonary arterial hypertension (PAH). The company listed on the Swiss Stock Exchange in 2000 and was later acquired by Johnson & Johnson in 2016.

Country
Denmark
Website
Adcendo ApS
Adcendo is a spinout from Copenhagen University dedicated to developing novel antibody-drug conjugates (ADCs) for the treatment of cancers. The company has a focus on cancer-associated targets that are professional internalizers and allow for superior selectivity via highly preferential cancer expression.
The company is building an ADC pipeline against uPARAP and other ideally suited ADC cancer targets. uPARAP is over-expressed in several forms of cancer, including soft-tissue sarcomas, osteosarcomas, and triple-negative breast cancers as well as by stromal cells in several high-prevalence cancers such as prostate, breast, and pancreatic cancer.

Country
Sweden
Aerocrine AB
Aerocrine was a medical technology company that develops and commercializes unique, easy-to-use and tailored products for the measurement of nitric oxide (NO) as an inflammatory marker for patients with inflammatory disorders, such as asthma. Aerocrine listed on the Nasdaq OMX Stockholm stock exchange in 2007 and was later acquired by Circassia Pharmaceuticals in 2015.

Country
USA
Aerogen Inc
Aerogen operated as a specialty pharmaceutical company that developed, manufactured and commercialized pulmonary drug delivery products. The company was acquired by Nektar in 2005.
Products

Country
Sweden
Affibody AB
Affibody was a Swedish biotech company focused on developing biopharmaceuticals based on its unique proprietary technology platforms Affibody® molecules and Albumod™.

Country
USA
Alba Therapeutics Corp
Alba’s platform was based on the discovery of zonulin, a protein that regulates cell barriers throughout the body. It enables development of novel therapeutics for a variety of inflammatory and immune mediated diseases, such as Celiac disease (gluten intolerance) and diabetes.

Country
Norway
Algeta ASA
Algeta was a Norwegian cancer drug maker that developed injectable radioactive oncology treatments. The company listed on the Oslo Stock Exchange in 2007 and was acquired by Bayer in 2014.
Products

Country
USA
Altimmune, Inc. (Immune Targeting Systems Ltd)
Altimmune is a clinical stage biotechnology company developing next-generation vaccines to address significant public health and biodefense needs. By leveraging specific attributes of its two independent and complementary vaccine delivery platforms, Altimmune can rapidly design vaccines against a wide range of disease targets, including respiratory diseases, chronic infections, and cancer.
Country
Switzerland
Apoxis SA
Apoxis was a Swiss company that focused on the discovery and development of therapeutics targeting cell death, inflammation and angiogenesis for the treatment of cancer and autoimmune disease. The company merged with TopoTarget A/S.

Country
US/Sweden
Website
Aprea Therapeutics
Aprea Therapeutics is a US/Swedish biopharmaceutical company that focuses on developing novel cancer therapies. The company specializes in the field of p53 biology and aims to harness the power of the p53 tumor suppressor protein to treat various types of cancers.
Aprea Therapeutics' lead drug candidate is APR-246, which is designed to reactivate the function of mutant p53 proteins commonly found in cancer cells. By restoring the normal function of p53, APR-246 has the potential to induce cancer cell death and inhibit tumor growth. The company is primarily focused on hematologic malignancies and solid tumors. Aprea Therapeutics conducts rigorous clinical trials and collaborates with leading academic institutions and research organizations to advance the development of its therapies.

Country
Germany
Website
Ariceum Therapeutics
Ariceum Therapeutics is a clinical stage radiopharmaceutical company focused on the diagnosis and precision treatment of certain neuroendocrine and other aggressive, hard-to-treat cancers such as small cell lung cancer (SCLC) and neuroblastoma.
Ariceum’s lead molecule, satoreotide, is a proprietary peptide derivative that binds to the somatostatin 2 receptor on certain tumor types. Satoreotide is an antagonist of the SSTR2 receptor. Upon decay of the radioactive molecule, the alpha or beta particle emitted will severely damage and ultimately kill the tumor cell. Other compounds in Ariceum’s pipeline will target different structures, and the targeting molecule may be anything from a small molecule to a large antibody.

Country
USA
Website
Aro Biotherapeutics
Aro Biotherapeutics is a biotechnology company pioneering the development of tissue-targeted genetic medicines with a platform based on a proprietary protein technology called Centyrins. The company is developing a wholly-owned pipeline of Centyrin-based therapeutic candidates and is working with industry partners to leverage Centyrins for tissue-specific targeting of therapeutics for a diverse set of diseases. The pipeline is targeting various genetic muscular diseases and autoimmune diseases.

Country
Switzerland
Arpida AG
Arpida was a Swiss biopharmaceutical company focused on the discovery and development of novel proprietary antibiotics to treat severe bacterial infections. The company listed on the Swiss Stock Exchange in 2005.

Country
Sweden
AstaCarotene AB
AstaCarotene was a Swedish company that focused on extraction of astaxanthin from cultures of the microalgae Haematococcus pluvialis.

Country
Sweden
BioCrine AB
BioCrine was a Swedish company formed around the scientific platform on -cell physiology and pato-physiology in relation to diabetes type I and II, which originated from a research group at the Karolinska Institute and Karolinska Hospital. The company merged with BioStratum, Inc.

Country
Sweden
BioFactor Therapeutics AB
BioFactor was a Swedish company that focused on the development of novel pharmaceutical approaches for the treatment of gastrointestinal disorder. The company merged with Melacure Therapeutics AB.

Country
Sweden
Biolipox AB
Biolipox was a Swedish company formed around the discovery of the metabolic pathway for arachidonic acid, which originated from a research group at the Karolinska Institute and Karolinska Hospital. The company merged with Orexo AB.

Country
USA
Bionaut Pharmaceuticals Inc.
Bionaut was a drug discovery company which focused on small molecule drug candidates

Country
Sweden
Biotage AB (Pyrosequencing AB)
Biotage focuses on genetic analysis and medical chemistry. Pyrosequencing listed on the Stockholm Stock Exchange in 2000 and later merged with Biotage.

Country
Sweden
Website
Bonesupport AB
Bonesupport is a medical technology company focused on developing and commercializing innovative products for the treatment of bone voids or gaps caused by trauma, infection, disease, or surgical procedures. The company specializes in the field of orthobiologics and aims to improve patient outcomes in musculoskeletal conditions.
Bonesupport's main product is Cerament, a range of synthetic bone graft substitutes that are designed to mimic the properties of human bone. Cerament is composed of a unique combination of hydroxyapatite and calcium sulfate, providing a scaffold for bone regeneration while also delivering antibiotics to help combat infection. Cerament have a proven ability to heal defects by remodeling to host bone in six to twelve months. Cerament is a fully developed product platform that is commercially available in the U.S. and Europe.

Country
Sweden
BrainCool AB (BeneChill Inc.)
BrainCool AB developed products within therapeutic hypothermia to reduce risk of injury after acute ischemic events, such as cardiac arrest, stroke, and traumatic brain injury. BrainCool acquired all assets and rights from BeneChill Inc. for shares. BrainCool is listed on Aktietorget.

Country
USA
Website
Carisma Therapeutics
Carisma Therapeutics is a biotechnology company specializing in the development of innovative immunotherapies for the treatment of cancer. The company focuses on engineering Chimeric Antigen Receptor (CAR) macrophages, a type of immune cell, to enhance their ability to recognize and eliminate cancer cells.
Carisma's proprietary technology involves genetically modifying macrophages to express CARs, which are designed to recognize specific tumor antigens. By targeting these antigens, CAR macrophages can effectively locate and destroy cancer cells in a targeted manner. The company's approach harnesses the unique characteristics of macrophages, which are natural immune cells with inherent abilities to engulf and destroy pathogens and abnormal cells. Carisma's CAR macrophages have the potential to overcome limitations associated with other cell-based immunotherapies, such as CAR-T cell therapies. The pipeline is composed of several programs, with the lead program targeting HER-2 expressing tumors.

Country
Sweden
Carmeda AB
Carmeda is a medical coating company, which engages in the development, manufacturing, and marketing of biocompatible coatings for medical devices. The company was acquired by Gore in 2005.

Country
USA
Cebix AB (Creative Peptides AB)
Cebix focused on the development of a disease-modifying replacement peptide for the treatment of complications associated with diabetes.

Country
Sweden
CePep AB
CePep was a Swedish company formed around the scientific platform of cell penetrating peptides exerting effects on intracellular signal transduction pathways. The company merged with Orexo AB.

Country
France
Cerenis Therapeutics Holding SA
Cerenis was focusing on the discovery, development and commercialization of breakthrough HDL-related therapies for the treatment of cardiovascular and metabolic diseases. The company’s lead program is a recombinant and optimized HDL. Cerenis listed on Euronext Paris in 2015.

Country
USA
ChemoCentryx Inc.
ChemoCentryx is a biopharmaceutical company focused on discovering, developing and commercializing orally-administered therapeutics to treat autoimmune diseases, inflammatory disorders and cancer. The company listed on Nasdaq in 2012.
Products

Country
Sweden
Clinical Data care in Lund AB
Clinical Data Care in Lund AB was a Swedish company that provided clinical trial services. The company merged with Clinitrac AB. After the merger, the combined entity was acquired by the larger clinical research organisation Trial Form Support AB.

Country
Sweden
Clinitrac AB
Clinitrac was a Swedish company with a technology focused on registration of patient data in clinical trials. The company merged with Clinical Data Care in Lund AB. After the merger, the combined entity was acquired by the larger clinical research organisation Trial Form Support AB.

Country
France
CLL Pharma S.A. (Synt:em)
Synt:em S.A. was a French company focused on computer-assisted rational drug design. The company merged with CLL Pharma, a pharmaceutical laboratory that is developing generic drugs and galenical formulations.

Country
Sweden
Conpharm AB
Conpharm provided therapeutics based on natural and synthetic substances from the plant Podophyllum emodi for treatment of inflammatory diseases.
Products

Country
USA
CoreValve SA
CoreValve developed trans-catheter aortic valve implantation platforms. The company was acquired by Medtronic in 2009.

Country
Switzerland
Cytos Biotechnology AG
Cytos was a biopharmaceutical company focused on the development of targeted immunotherapies with a VLP B-cell vaccines platform. The company listed on the Swiss Stock Exchange in 2002 and was combined with Kuros Biosurgery to create Kuros Biosciences in 2015.

Country
Sweden
Website
Doctrin
Doctrin, founded in 2016, aims to facilitate healthcare providers to digitize the patient journey. The company has developed a cloud-based B2B system for healthcare providers to collect medical history from patients before a medical consultation. In addition, Doctrin provides digital solutions for doctors’ consultations to increase patient involvement and facilitate doctor-patient communication. The development will continue with AI-based solutions.

Country
USA
Dynavax Technologies
Dynavax wasa clinical-stage biopharmaceutical company, which used Toll-like Receptor (TLR) biology to discover and develop novel vaccines and therapeutics in the areas of infectious and inflammatory diseases and oncology. The company listed on Nasdaq in 2004.

Country
Sweden
Website
Elsa Science AB
Elsa Science is a digital health tech company aiming at improving care for patients with chronic diseases, starting with rheumatic arthritis (RA). The company was founded based on research from professors Lars Klareskog (an authority in the rheumatology field) and Lars Alfredsson. Elsa provides a platform that holds several functionalities such as a digital companion, remote patient monitoring, optimal personalized treatment, and prognostic models.

Country
USA
Esperion Therapeutics Inc
Esperion Therapeutics was a biopharmaceutical company primarily focused on the research and development of therapies to regulate high-density lipoprotein cholesterol, or HDL-C. The company listed on Nasdaq IN 2000.

Country
Sweden
EsyTech AB
EsyTech was a Swedish medical technology company focused on the research, development, manufacturing and commercialization of instrumentation and related products intended for chemical analysis or other types of measurements. The company merged with Pyrosequencing.

Country
USA
Ferrokin Biosciences Inc.
Ferrokin developed an iron chelator for the treatment of iron-overload in patients with transfusion-dependent hereditary and acquired refractory anemia. The company was acquired by Shire in 2012.

Country
USA
Five Prime Therapeutics Inc.
FivePrime is discovering and developing innovative antibody and protein therapeutics using its comprehensive discovery platform. The company listed on Nasdaq IN 2013.

Country
Canada
Website
Fusion Pharmaceuticals Inc.
Fusion Pharmaceuticals is a biopharmaceutical company that focuses on developing targeted alpha therapies (TATs) for the treatment of cancer. The company utilizes a unique approach that combines the precision of monoclonal antibodies with the potent cell-killing properties of alpha-emitting radioisotopes. Fusion Pharmaceuticals' technology platform involves linking highly specific antibodies to alpha particle-emitting isotopes. This enables the targeted delivery of radiation directly to cancer cells while sparing healthy tissues, thereby maximizing the therapeutic effect and minimizing side effects.
The company's pipeline includes a range of candidates designed to target different types of solid tumors. These candidates are being developed for both primary and metastatic cancers, to improve patient outcomes and potentially offer new treatment options where traditional therapies have limitations.
In March 2024, it was announced that AstraZeneca would acquire Fusion Pharmaceuticals for a total transaction value of approximately $2.4 billion including the CVR.

Country
Canada
Genizon BioSciences Inc. (Global Genomics)
Global Genomics was a Swedish company with a patented technology for rapid and accurate global expression analysis of genes. The company merged with Genizon BioSciences.

Country
France
GenSight Biologics S.A.
GenSight was a clinical-stage biotechnology company discovering and developing novel therapies for mitochondrial and neurodegenerative diseases of the eye and central nervous system. GenSight’s first program is a gene therapy for Leber Hereditary Optic Neuropathy, a rare genetic disorder, in which patients lack an enzyme necessary for energy production leading to gradual vision loss and blindness. The company has received orphan drug designation in EU and US for this program. Gensight is listed on Euronext Paris.
Country
Sweden
Website
Gesynta Pharma
Gesynta Pharma is a Swedish pharmaceutical company that develops unique drug candidates with the aim to reduce harmful inflammation and pain, thereby providing safe and efficacious treatments for several serious diseases. Based on research originating from Karolinska Institutet, their lead product is a novel treatment for endometriosis.

Country
Sweden
Glionova AB
Glionova Therapeutics is a biopharmaceutical company focused on developing new therapies for cancer. The Company’s lead candidate is a first-in-class, orally available oncolytic small molecule, currently being developed as a potentially disease modifying therapy for glioblastoma by targeting specific vulnerabilities in tumor cells.

Country
Sweden
Gyros AB
Gyros manufactures laboratory instruments, consumables, kits and software to maximize laboratory productivity by miniaturizing and automating immunoassays at nanoliter-scale.

Country
Germany
Website
HelloBetter
HelloBetter is a digital health company that offers evidence-based online mental health and well-being programs. The company aims to make mental health support accessible, convenient, and effective for individuals seeking help. HelloBetter provides a wide range of self-help and therapeutic programs that are designed to address various mental health conditions, including anxiety, depression, stress, and insomnia.
By leveraging technology and evidence-based interventions, HelloBetter aims to bridge the gap in mental healthcare by providing accessible and scalable solutions. The company's approach combines the convenience of online delivery with the effectiveness of evidence-based therapies, offering individuals an opportunity to improve their mental health and well-being flexibly and affordably.

Country
Denmark
Website
Hemab Therapeutics
Hemab Therapeutics is a Danish biotech company focused on developing novel treatments for rare and underserved coagulation disorders. Hemab is building a pipeline of bi-specific antibodies targeting various underserved bleeding disorders. The company's lead asset is in preclinical development for Glanzmann’s thrombasthenia (GT), a rare bleeding disorder and makes use of a well-understood and clinically validated mechanism utilizing supraphysiological levels of factor VII to treat bleeding episodes in GT.

Country
France
Hybrigenics S.A.
Hybrigenics was a bio-pharmaceutical company focused on research and development of new targets and therapies against proliferative diseases. The company listed on Euronext Paris in 2007.
Country
Germany
IDEA AG
IDEA’s proprietary technology focused on enabling non-invasive delivery of drugs through the skin.

Country
USA
Website
InCarda Therapeutics Inc.
InCarda Therapeutics is a clinical stage biotechnology company developing an inhaled therapy to treat cardiovascular conditions and diseases, the lead product under development is an inhaled therapy to treat paroxysmal atrial fibrillation, a widespread atrial arrhythmia. InCarda employs a de-risked approach by using approved drugs with a long history of efficacy and safety in a new dosing paradigm.

Country
Finland
Inion Oy
Inion was a Finnish medical device company that developed non-metallic biodegradable implants to improve surgical solutions for reconstructive surgery, orthopedics, sports medicine and dental surgery. The company listed on the London Stock Exchange in 2004.

Country
Sweden
Website
Intervacc
Intervacc AB is a Swedish public company active in the biotechnology sector. The company develops new vaccines based on fusions of recombinant proteins to meet the increasing need for effective vaccines in animal health care, with a very good safety profile. The lead product is Strangvac, a vaccine targeted against the equine disease strangles.

Country
Germany
Jerini AG
Jerini was an orphan drug company focused on novel peptide-based drugs, with lead product Firazyr targeting Hereditary Angioedema. The company listed on the Frankfurt Stock Exchange in 2005 and was acquired by Shire in 2008.
Products

Country
Sweden
Karo Pharma (Karo Bio)
Karo Pharma developed and marketed drugs for unmet medical needs to pharmacies, grocery stores and directly to hospitals. The company started as a biotech-company with several candidates in pre-clinical phase based on research on nuclear receptors as target proteins. Today the company has added several approved pharmaceutical products to complement its R&D Pipeline.

Country
The Netherlands/USA
Website
Kivu Bioscience
Kivu Bioscience is a biotech company developing next-generation antibody-drug conjugates to deliver best-in-class therapeutics against several different indications in oncology. The company’s proprietary linker-payload technology delivers enhanced safety and efficacy, minimizing off-target effects to improve patient outcomes.

Country
Denmark
Lica Pharmaceuticals A/S
Lica Pharmaceuticals provided novel and unique compounds for the treatment of parasitic and bacterial diseases.

Country
Sweden
LTB4 Sweden AB
LTB4 developed a naturally occurring molecule stimulating the innate immunity system to target diseases such as infections and cancer.

Country
Switzerland
Lumavita AG
Lumavita was a Swiss specialty pharmaceutical company that focused on the development and marketing of new drugs to address infections for women’s health.
Products

Country
USA
Website
Mahzi Therapeutics
Mahzi is a start-up company co-founded by HealthCap, Ultragenyx, and CEO Yael Weiss. Mahzi focuses on developing therapeutics for underserved rare genetic neurodevelopmental disorders. Mahzi is working with genetic treatment modalities such as gene therapy and RNA-based therapeutics. The goal is to choose the best therapeutic approach for each individual disease and to advance that potential medicine as rapidly as possible.

Country
USA
Maxim Pharmaceuticals, Inc.
Maxim was a US-based listed biopharmaceutical company developing cancer and vaccine products.
Products

Country
Sweden
Medicarb AB
Medicarb developed biocompatible coating technologies and merged with Carmeda, which was acquired by Gore in 2005.

Country
Sweden
Melacure Therapeutics AB
Melacure was a Swedish company that focused on melanocortin receptor biology and pharmacology.

Country
Sweden
Millicore AB
Millicore developed MEMS-based (Micro-Electro-Mechanical Systems) intelligent disposable surgical products to increase the quality and decrease the cost of care. Millicore was acquired by Medela in 2010.

Country
Sweden
MIPS AB
MIPS is a global leader in helmet safety with a patented technology designed to reduce rotational forces to the brain during impact. Helmets integrated with MIPS’ safety solutions are marketed globally by major helmet brands in sport segments such as bicycle, snow, motorcycle and equestrian. Mips is listed on Nasdaq Stockholm Exchange.

Country
Sweden
Modus Therapeutics
Modus Therapeutics, located in Stockholm, Sweden, is developing a novel treatment targeting the vaso-occlusive crises associated with sickle-cell disease (SCD). SCD is a devastating rare genetic red blood cell disorder. It is characterized by abnormal hemoglobin resulting in anemia and sickling of the red blood cells that obstruct the blood flow.

Country
Sweden
Neoventa Medical AB
Neoventa is a provider of innovative fetal monitoring solutions and services that improve obstetric care.

Country
USA
NephroGenex (BioStratum, Inc.)
NephroGenex Inc. is developing Pyridorin™ (pyridoxamine dihydrochloride) as a treatment to slow the progression of diabetic kidney disease. Pyridorin was acquired for shares from BioStratum, Inc. NephroGenex listed on Nasdaq in 2014.

Country
Switzerland
Neuro3D SA
Neuro3D developed innovative treatments for psychiatric disorders such as schizophrenia, depression and anxiety. The company was acquired by Evotec in 2007.

Country
Sweden
Newron Pharmaceuticals SpA (NeuroNova AB)
Newron is a biopharmaceutical company with focus on therapies for the Central Nervous System in indications such as Parkinson’s disease, neuropathic low back pain and other cognitive disorders. The company acquired NeuroNova AB in 2012 and is listed on the Swiss Stock Exchange.

Country
Finland
Nexstim Oy
Nexstim focused on commercializing a brain scanning technology enabling pre-surgical mapping, neurodiagnostics, as well as neurotherapy applications such as stroke rehabilitation. Nexstim listed on Nasdaq OMX Stockholm and Helsinki stock exchanges in 2014.

Country
France
NicOx S.A.
NicOx is a French ophthalmic company which listed on Euronext Paris in 1999.
Products

Country
USA
Nobex Corp.
Nobex focused on the modification of peptide drugs for improved clinical performance and delivery into the body.

Country
Norway
Nordic Nanovector AS
Nordic Nanovector AS is a clinical stage biotechnology company developing radiopharmaceuticals for hematological cancer patients. Nordic Nanovector’s lead product Betalutin is currently being investigated in Non-Hodgkin’s lymphoma (NHL) and has received orphan drug designation in EU and US. Nordic Nanovector listed on the Oslo Stock Exchange in 2015

Country
Sweden
Nordic Vision Clinics AS (Optivy AB)
Nordic Vision Clinics AS is a chain of laser eye surgery clinics in Sweden under the name Globen Ögonklinik.

Country
USA
Nucleonics, Inc.
Nucleonics was a US biotechnology company focused on the development of novel RNA-interference based therapeutics for viral diseases (such as hepatitis B, hepatitis C and HIV) as well as other diseases.

Country
USA
Odyssey Thera Inc.
Odyssey Thera’s proprietary platform technology, protein-fragment complementation assay (PCA), is designed to optimize the predictability and speed of drug discovery and development to find treatments for cancer and other diseases.

Country
Sweden
Website
Oncopeptides AB
Oncopeptides is a clinical stage pharmaceutical company developing oncology therapies centered on peptidase targeting. Melflufen, Oncopeptides lead drug compound, is a peptide-drug conjugate that targets aminopeptidases and releases alkylating agents into tumor cells. The main therapeutic area is relapsed/refractory multiple myeloma. Orphan Drug Designation for melflufen in the treatment of multiple myeloma has been granted by the European Medicines Agency, EMA. Melflufen has been granted full approval by EMA and the Medicines and Healthcare Products Regulatory Agency, MHRA, in the UK. Oncopeptides is listed on Nasdaq Stockholm Exchange.
Products

Country
Sweden
Website
Oncorena
Oncorena is a Swedish pharma company developing a potentially curative therapy for metastatic kidney cancer based on orellanine, a toxin from the Cortinarius family of mushrooms.

Country
France
Onxeo S.A. (TopoTarget A/S)
Onxeo was a clinical stage biopharmaceutical company focused on orphan oncology, created by the merger between Topotarget and Onxeo. Its lead drug candidate, Beleodaq, has shown positive results in the treatment of hematological malignancies and solid tumors, obtained by both mono- and combination therapy and has received orphan drug designation in EU and US for Peripheral T-Cell Lymphoma. Onxeo is listed on Euronext Paris and Nasdaq OMX Copenhagen stock exchanges.
Products

Country
USA
OraPharma Inc.
OraPharma Inc. offered oral healthcare for the treatment of periodontal disease. The company was listed on Nasdaq up to its acquisition by Johnson & Johnson.
Products

Country
USA
Orchid BioSciences Inc.
Orchid BioSciences focused on the development and commercialization of genetic diversity technologies, products and services. The company listed on Nasdaq in 2000 and later changed its name to Orchid Cellmark. Orchid Cellmark was acquired by Laboratory Corporation of America in 2011.

Country
Sweden
Orexo AB
Orexo was a specialty pharmaceutical company developing improved treatments using its proprietary drug delivery technology. The company has a portfolio of revenue generating EU and US approved products currently marketed under license and a pipeline of several reformulations of approved compounds for areas of unmet medical need. Orexo listed on the Nasdaq OMX Stockholm Stock Exchange in 2005.
Products

Country
Sweden
OxThera AB
OxThera is a spin-out company from Q-Med AB and is developing products using its cell- and enzyme-based proprietary technologies for metabolic disorders based on excess oxalate, a primary risk for kidney stone formation.

Country
Sweden
Personal Chemistry AB
Personal Chemistry was a Swedish company that had developed a technology by which microwaves used to catalyze chemical reactions. The company merged with Pyrosequencing.

Country
Denmark
Pharmexa A/S
Pharmexa A/S was a Danish vaccine company which listed on the Copenhagen Stock Exchange in 2000 and was later merged with Affitech AS.

Country
USA
Pharmion Corp.
Pharmion focused on acquiring, developing and commercializing innovative products for the treatment of hematology and oncology patients. The company listed on Nasdaq in 2003 and was later acquired by Celgene.
Country
Switzerland
Website
Positrigo
Positrigo is a Swiss medical technology company focused on accessible, ultra-compact, and affordable positron emission tomography (PET) brain imaging. Founded as a spin-off from ETH Zurich, the company has developed NeuroLF, a compact, dedicated brain PET scanner designed to support the diagnosis of neurological conditions such as Alzheimer’s, Parkinson’s, and brain tumors, combining compact size with high image quality and patient comfort. Unlike traditional PET systems, NeuroLF is space-efficient, cost-effective, and easy to integrate into clinical settings. NeuroLF has received both FDA clearance and CE marking, enabling broader adoption across the U.S. and Europe.

Country
Belgium
Website
Precirix
Precirix is a clinical-stage spin-off company from Vrije Universiteit Brussel, developing novel radiopharmaceuticals to treat cancer. The proprietary technology combines small single-domain antibody fragments, called nanobodies, with radioactive isotopes to treat cancer.
The company’s lead product, CAM-H2, is being evaluated in metastatic HER2-positive cancer. Patients with tumors that overexpress HER2 can benefit from effective targeted treatments and have a poor prognosis when the cancer spreads. CAM-H2 aims to effectively irradiate cancer lesions while sparing healthy tissue, based on its unique technology platform that leverages the favorable tissue distribution of sdAbs.

Country
USA
Website
Pretzel Therapeutics
Pretzel Therapeutics is a biotechnology company developing therapeutics to reverse mitochondrial dysfunction by leveraging a deep mechanistic understanding of mitochondrial biology. The company aims at genetic drivers of mitochondrial dysfunction relevant to a wide range of mitochondrial disorders. The company is based on academic research spun out of the Karolinska Institute, Gothenburg University, and the University of Cambridge.

Country
Ireland
Website
Priothera Ltd.
Priothera is a start-up biotech company looking to develop mocravimod, a S1P receptor modulator, for the treatment of high-risk acute myeloid leukemia (AML) patients who are undergoing hematopoietic stem cell transplantation (HSCT). Recent long-term data from a proof-of-concept study in patients with hematological cancers treated with HSCT indicates that mocravimod may be an attractive novel treatment for addressing an unmet medical need for these patients.

Country
Germany
ProCorde AG
ProCorde was a German cardiovascular-focused company that merged with Trigen Ltd., a UK-based company developing drugs for the treatment of cardiovascular diseases with a focus on thrombosis.

Country
United Kingdom
Prolifix Ltd.
Prolifix was a UK-based drug discovery-company focusing its activities on developing new pharmaceuticals which inhibit the cell cycle. The company merged with TopoTarget A/S.

Country
USA
Prothelia Inc.
Prothelia was a US based private biotech company founded in 2007. Prothelia’s lead program is a novel protein therapy for the treatment of congenital muscular dystrophy type 1A, a rare disease affecting approximately 4,000 patients.

Country
USA
PTC Therapeutics Inc.
PTC focuses on the discovery, development and commercialization of orally administered, proprietary small molecule drugs targeting an area of RNA biology. The company listed on NASDAQ in 2013.
Products

Country
USA
Website
Pulmonx Corp.
Pulmonx is focused on developing and marketing minimally-invasive medical devices and technologies for the diagnosis and treatment of emphysema and other severe lung diseases. Pulmonx's flagship product is the Zephyr Endobronchial Valve (EBV), a minimally invasive device designed to treat severe emphysema. The Zephyr EBV is inserted into the airways of the lungs using a bronchoscope, where it helps to redirect airflow to healthier regions of the lung. By doing so, it can improve breathing, reduce shortness of breath, and enhance overall lung function. Pulmonx products are commercially available in more than 25 countries.

Country
Sweden
Q-Med AB
Q-Med marketed a portfolio of products developed using the company’s proprietary technology based on stabilized hyaluronic acid. The company listed on the Stockholm Stock Exchange in 1999 and was later acquired by Swiss pharmaceutical company Galderma.
Products

Country
USA
Rainier Therapeutics Inc.
Rainier Therapeutics was developing a monoclonal antibody for the treatment of genetic orphan and oncology diseases. Development programs included achondroplasia, a rare skeletal condition, and metastatic bladder cancer patients who already have failed first-line chemotherapy. Rainier was acquired by Fusion Pharmaceuticals in 2021.

Country
USA
Website
Rampart Biosciences
Rampart Biosciences is a US company developing a new class of gene therapies to circumvent the limitations of available technologies. Using a non-viral delivery system to deliver DNA-based vectors, Rampart aims to increase the potency and safety of gene therapy, while also enabling re-dosing. The primary indications are genetically driven rare diseases.

Country
USA
Renovis Inc.
Renovis developed drugs to treat neurological diseases and disorders. The company listed on Nasdaq in 2004 and was acquired by Evotec in 2008.

Country
United Kingdom
Renovo Ltd.
Renovo developed drugs to prevent scars and accelerate wound healing in the skin. The company listed on the London Stock Exchange in 2006.

Country
Sweden
Resistentia Pharmaceuticals AB
Resistentia was a Swedish research-based pharmaceutical company focusing on the development of vaccines aimed at preventing allergy.

Country
USA
ReVENT Medical Inc. (ReVent Medical International B.V.)
ReVENT Medical has developed the ReVENT Sleep Apnea System, which comprises minimally invasive, biocompatible implants targeting tongue based Obstructive Sleep Apnea (OSA). The device is CE marked for the European market.

Country
Sweden
RSPR Pharma AB
RSPR Pharma was a Swedish pharmaceutical company developing an oral anti-asthmatic drug providing mast cell control by inhibiting both induced and basal release of asthma mediators.
Asthma is an inflammatory condition affecting some 300 million people worldwide and despite gold standard therapy (inhaled corticosteroids, which do not target mast cells) only 50% of patients reach asthma control.

Country
Sweden
Sequenom Inc. (Eurona AB / Gemini Genomics plc)
Eurona AB was a clinical genetics company with a proprietary genotyping technology. Eurona was merged into Gemini Genomics, which listed on NASDAQ in 2000 and later was merged with Sequenom.

Country
USA
Sopherion Therapeutics Inc.
Sopherion was a biopharmaceutical company which acquired, discovered, and developed targeted anti-cancer agents. All assets have been acquired by Zoticon.

Country
France
SpineVision S.A.
SpineVision was a medical technology company developing a comprehensive range of spinal implants. The spinal implants are used for treatment of spinal disorders, including deformities, degenerative disc disease, tumors and traumas. The company has several products on the market and distributes them throughout the world.

Country
USA
Website
Spruce Biosciences
Spruce Biosciences is a private US-based clinical-stage biopharmaceutical company developing novel therapies for rare endocrine disorders. The company focuses on creating treatments that target the underlying mechanisms of these disorders to provide meaningful improvements for patients. Spruce’s lead product candidate, tildacerfont (SPR001), is an investigational oral therapy being developed for the treatment of congenital adrenal hyperplasia (CAH), a rare genetic disorder characterized by the overproduction of androgens, which can lead to a variety of symptoms and health complications. Tildacerfont aims to normalize androgen levels by selectively blocking the action of adrenocorticotropic hormone (ACTH). Tildacerfront also has a potential role in the treatment of polycystic ovary syndrome.

Country
Ireland
Website
SynOx Therapeutics Ltd
Synox, based in Ireland, is a spin-out from UK-based oncology company Celleron and is jointly formed by Celleron, the investors, and Roche. The company is developing emactuzumab, an antibody licensed from Roche, targeting a rare soft tissue tumor called tenosynovial giant cell tumor (TGCT). TGCT is a rare disease that causes significant pain and disability, often affecting joints in the lower extremities, currently with limited treatment options.
The monoclonal antibody emactuzumab is a potent, specific inhibitor of CSF-1R with potential as a therapeutic platform targeting serious macrophage-driven inflammatory, fibrotic, and neovascular diseases.

Country
Norway
Targovax AS (Oncos)
In 2015, Targovax acquired Oncos in an all-stock transaction, creating a new Nordic leader in immuno-oncology. The new combined company Targovax is targeting two complementary approaches to cancer immunotherapy: a cancer vaccine platform developed for patients with RAS-mutated cancers and an immunotherapy platform based on engineered oncolytic viruses armed with potent immune-stimulating transgenes for patients with solid tumors. Targovax is listed on Oslo Børs.

Country
Denmark
Technolas Perfect Vision GmbH
Technolas Perfect Vision was a leading ophthalmology laser company, formed through a joint venture between Bausch + Lomb, and 20/10 Perfect Vision AG. The company was acquired by Bausch + Lomb in 2013.

Country
USA
Tengion Inc
Tengion focused on regenerative medicine and the development of its Organ Regeneration Platform™ to harness the intrinsic regenerative pathways of the body to regenerate a range of native-like organs and tissues. The company listed on NASDAQ in 2010.

Country
Netherlands
Tisbury Pharmaceutical
Tisbury Pharmaceutical Inc (Tisbury Pharmaceuticals BV). is a pharmaceutical company developing a first-in-class drug to treat glaucoma. Its primary development target is patients with open angle glaucoma and ocular hypertension patients.

Country
Sweden
Website
Tribune Therapeutics AB
Tribune Therapeutics is a pre-clinical stage biopharmaceutical company co-founded by HealthCap. Tribune is developing a fusion protein to treat patients with fibrotic disease. The fusion protein targets the CCN protein family, which is a group of signaling proteins central to the development of fibrosis. CCN2 (also known as CTGF) has been studied extensively in the context of fibrosis as a downstream signaling mediator of TGFβ activation.

Country
Germany
Trigen Ltd.
Trigen was a UK-based biotechnology company that developed drugs for the treatment of cardiovascular diseases with a focus on thrombosis. The company merged with ProCorde, an early-stage German cardiovascular company.

Country
Sweden
Trimb Healthcare AB
Trimb is focused on the development and sales and marketing of prescription free pharmaceuticals (OTC) and personal care products. The Company has its own go-to-market organization in Northern Europe and in addition, select products are sold internationally through distributors.

Country
Sweden
Tripep AB
Tripep focused on the development of new types of therapies using its protein polymer inhibitor platform technology, with an initial focus on HIV. The company listed on the Stockholm Stock Exchange in 2000.

Country
USA
Ultragenyx Pharmaceuticals Inc.
Ultragenyx focuses on developing therapeutics for rare (orphan) diseases. Following its formation, the company rapidly built a diverse portfolio of orphan drug product candidates and listed on NASDAQ in 2014.

Country
Sweden
Ultrazonix Holding AB
Ultrazonix was a Swedish medical technology company that focused on the development of high-intensive focused ultrasound products primarily for the treatment of low back pain. The company merged with BoneSupport AB.

Country
Sweden
Vägakuten AB
Vägakuten was a Swedish company with a strategy to establish highly accessible roadside medical stations.

Country
Sweden
Website
Vicore Pharma
Vicore Pharma is a Swedish biopharmaceutical company focused on the development of innovative therapies for serious lung disorders, including idiopathic pulmonary fibrosis (IPF). In 2018, listed company Vicore merged with HealthCap startup INIM Pharma, to form a company with the potential to become a leader in the field of rare lung diseases.
The company's primary candidate is VP01 (C21), an orally administered small molecule that targets the angiotensin II type 2 receptor (AT2R). VP01 is being developed as a potential treatment for IPF, a progressive and debilitating lung disease characterized by scarring of the lung tissue that occurs primarily in middle-aged and elderly adults. By leveraging its expertise in AT2R modulation, the company aims to provide new therapeutic options for patients in need, potentially improving their quality of life and prognosis.

Country
Denmark
Vir A/S
Vir focused on the development of a surface plasmon resonance technology.

Country
Sweden
Visual Bioinformatics AB
Visual Bioinformatics was a Swedish company that developed novel and innovative software solutions for storage, retrieval and interpretation of data generated through functional genomics technologies. The company was merged with Affibody AB.

Country
France
Website
Vivet Therapeutics
Vivet Therapeutics is a French biotechnology company developing gene therapy for rare liver diseases with a high unmet medical need, including Wilson Disease, primary familial intrahepatic cholestasis type 2 (PFIC2), and citrullinemia type 1 (CTLN1). The company focuses on rare genetic diseases that impact the liver and aims to provide innovative and potentially curative treatments for patients with limited therapeutic options. The company’s development strategy is to target the liver with novel synthetic adeno-associated virus vectors to introduce therapeutic genes into the liver cells of the patients. By correcting or compensating for the genetic defects underlying these disorders, the company's gene therapies aim to restore normal liver function and improve patient outcomes.
Vivet’s lead program, VTX-801, is an investigational gene therapy for Wilson disease, a rare genetic disorder characterized by impaired copper metabolism. VTX-801 aims to restore the liver's ability to properly regulate copper levels, potentially preventing or reversing the progression of the disease.

Country
Sweden
Wilnor AB
Wilnor was a Swedish company focused on the development and marketing of software for industrial and clinical laboratories.

Country
Sweden
Wilson Therapeutics AB
Wilson Therapeutics was an orphan biopharmaceutical company, based in Stockholm, Sweden, that developed novel therapies for patients with Wilson Disease. Wilson was listed on Nasdaq Stockholm Exchange and was acquired by Alexion in 2018.
Country
Sweden
XCounter AB
XCounter developed a digital x-ray imaging technology enabling single photon detection and determination of a photon’s individual energy. The company listed on the London Stock Exchange’s AIM market in 2006.

Country
Sweden
Xenerate AB
Xenerate was a Swedish company developing a technology to enable artificial vascular prosthesis, such as stents and grafts, to be covered by endothelial cells.

Country
USA
Xeris Biopharma Holdings, Inc (Strongbridge)
Strongbridge Biopharma was a global commercial-stage biopharmaceutical company focused on the development and commercialization of therapies for rare diseases with significant unmet needs. The main therapeutic focus was on rare neuromuscular and endocrine diseases such as Primary Periodic Paralysis and Cushing’s syndrome. The company listed on NASDAQ in 2015 and was acquired for shares by Xeris Pharmaceuticals in 2021.

Country
United Kingdom
York Pharma Plc (Charterhouse)
Charterhouse Therapeutics was an early stage drug discovery company with focus on research and development of anti-viral drugs based on a naturally occurring molecular structure, the cyclopentenone ring of certain prostaglandins. The company merged with York Pharma Plc.
All Products
Devices
Pharmaceuticals
Digital Therapies
Launched year
2008
Company
Abstral
Abstral® is a drug that provides fast and effective treatment of breakthrough pain in cancer patients. It is based on Orexo’s sublingual tablet technology and the analgesic fentanyl.
More from Orexo AB

Launched year
2002
Company
Aeroneb® Pro
The Aeroneb® Pro is Aerogen’s flagship nebulizer for aerosol therapy. Aeroneb® Pro is a proven nebulizer for effectively treating mechanically ventilated patients . The Aeroneb® Pro is a reusable, multi-patient use nebulizer which is suitable for hospital environments where the appropriate sanitization facilities are available.
This autoclavable nebulizer provides effective dose delivery of physician- prescribed inhalation solutions for infants through adults in both on and off ventilator applications. Therapy can be delivered using an aerosol mask or a mouthpiece in an off ventilator application, see the Instructions for Use manual for more information.
Utilizing Aerogen’s OnQ™ Aerosol Generator, the Aeroneb® Pro produces a fine particle, low velocity aerosol without environmentally unfriendly propellants, inefficient compressors or costly ultrasonic elements. The Aeroneb® Pro nebulizer is a vibrating mesh nebulizer in a class of its own.
Launched year
2002
Company
Arestin
Arestin® (minocycline HCl) is a subgingival sustained-release product containing the antibiotic minocycline hydrochloride incorporated into a bioresorbable polymer for professional subgingival administration into periodontal pockets.
Launched year
2014
Company
Beleodaq
Beleodaq® (belinostat) is a histone deacetylase inhibitor indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma (PTCL). This indication is approved under accelerated approval based on tumor response rate and duration of response.
Launched year
2010
Company
Ceplene
Ceplene® (histamine dihydrochloride) is an immune-enhancing product which is intended to use in combination with interleukin-2 (IL-2) as a remission maintenance treatment of acute myeloid leukemia.
Indication
Bone cysts; bone defects caused by various reasons
CE mark received
2009
FDA clearance received
2005
Company
CERAMENT® Bone Void Filler
The CERAMENT™|BONE VOID FILLER is ceramic bone void filler intended only for orthopedic applications as a filler for gaps and voids that are not intrinsic to the stability of the bony structure. It provides bone void filler that resorbs and is replaced by bone during the healing process. The product is intended to be injected into bony voids or gaps in the skeletal system, i.e. extremities, spine, and pelvis.
More from Bonesupport AB
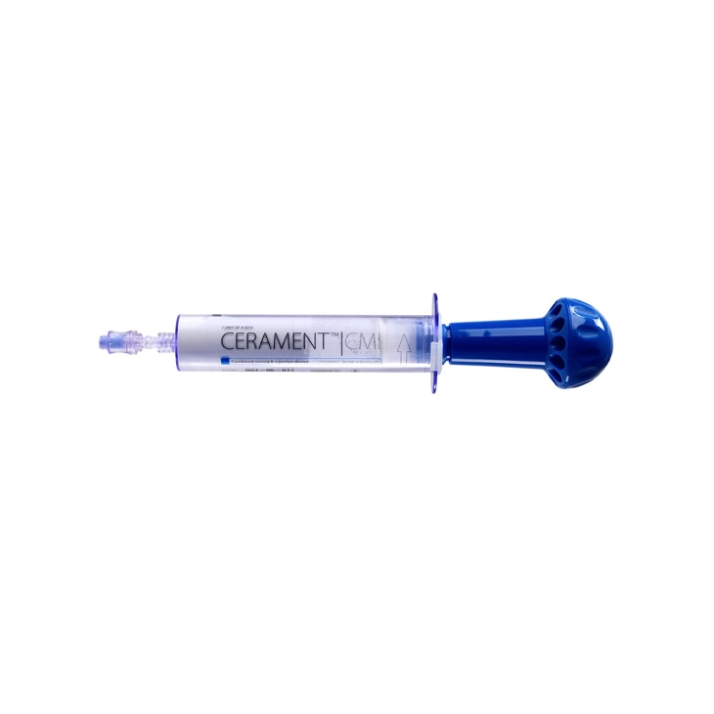
CERAMENT® Gentamicin and Supporting System
The CERAMENT® Gentamicin is an injectable formulation of synthetic bioceramic bone substitute and the antibiotic drug gentamicin. The biomaterial consists of calcium sulphate and hydroxyapatite combined with liquid Iohexol creates a highly injectable, osteoconductive and bioactive material. It is designed to mimic human cancellous bone by providing short term resorbable support for the fracture or bone void, and longer term osteoconductive support which enhances new bone growth.
More from Bonesupport AB
CERAMENT™ | SPINE SUPPORT
The CERAMENT™|SPINE SUPPORT is an injectable ceramic bone substitute intended to use in the stabilization of vertebral compression fractures in the vertebral body. It is the only fully injectable biological material with C.E. MARK for the treatment of Vertebral Compression Fractures offering clinically demonstrated bone remodelling / healing.
More from Bonesupport AB
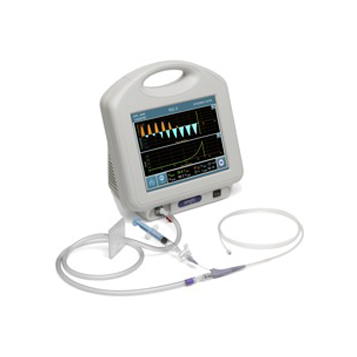
Chartis Pulmonary Assessment System
The Chartis System is a unique assessment tool that provides precise flow and pressure readings at the lobar or segmental level. This information enables the physician to make assessments regarding the level of collateral ventilation, or inter-lobar airflow in the lungs. Collateral ventilation (CV) can limit the effectiveness of endobronchial lung-volume reduction therapy, and is therefore an important predictor of EBV treatment success.
By directly detecting the presence of CV in the targeted lobes of the lungs, this innovative technology platform empowers physicians to plan data-driven treatment with Zephyr valve therapy.
More from Pulmonx Corp.

Launched year
2021
Company
Chronic Pain
The ratiopharm Chronic Pain course was developed by experts from science, psychotherapy and psychology, incorporating input from people who suffer from chronic pain. Patients will acquire effective strategies for dealing with chronic pain: learn how to change the way that pain affects you through acceptance, discover what’s truly important to you in life and how you can apply this knowledge to reshape your everyday life. The effectiveness of the course has been examined and confirmed in a randomised controlled trial where the participants reported significantly less pain interference.
More from HelloBetter
Launched year
2018
Company
Crysvita
Crysvita (burosumab) is an anti-FGF23 fully human monoclonal antibody for the treatment of X-linked hypophosphatemia (XLH) in children. XLH is a rare, chronic progressive musculoskeletal disorder that affects children and adults.
More from Ultragenyx Pharmaceuticals Inc.

Launched year
2021
Company
Diabetes and Depression
Diabetes and Depression is the first online therapy course for the treatment of depressive symptoms in people with type 1 and 2 diabetes. The course is based on cognitive behavioral therapy and patients learn about their disorder, strengthen their problem-solving skills, and learn new behaviors as well as long-term strategies to prevent relapses. The RCT demonstrated that 62 percent of participants showed a substantial improvement in depressive symptoms.
More from HelloBetter

Launched year
2020
Company
DOJOLVI
Dojolvi™ (UX007/triheptanoin) is the first FDA-approved therapy for the treatment of long-chain fatty acid oxidation disorders, a group of rare, lifelong and life-threatening genetic disorders in which the body is unable to convert long-chain fatty acids into energy.
More from Ultragenyx Pharmaceuticals Inc.
Launched year
2009
Company
Edluar
Edluar® (zolpidem tartrate) is based on Orexo’s sublingual tablet technology and the active substance zolpidem and offers treatment for short-term insomnia. The Edluar tablet is placed under the tongue where it rapidly dissolves and the active substance is absorbed through the mucous membrane.
More from Orexo AB
Launched year
2010
Company
Femifect
FemiFect® (pentamycin), a broad-spectrum macrolide antibiotic, is approved for the treatment of vaginitis.
Launched year
2009
Company
Firazyr
Firazyr® (icatibant) Injection for the treatment of acute attacks of a rare condition hereditary angioedema (HAE) in people ages 18 years and older.
Launched year
2008
Company
Innohep
Innohep® (tinzaparin sodium) is a low molecular weight heparin (LMWH) with antithombotic properties indicated for the treatment and prophylaxis of deep vein thrombosis and pulmonary embolism.
More from Pharmion Corp.
Launched year
2017
Company
MEPSEVII
MEPSEVII™ (vestronidase alfa) is an enzyme replacement therapy indicated in pediatric and adult patients for the treatment of Mucopolysaccharidosis VII (MPS VII, Sly syndrome).
More from Ultragenyx Pharmaceuticals Inc.
Company
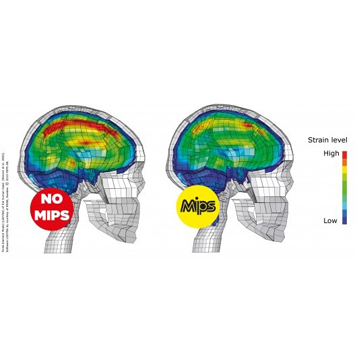
Mips Brain Protection System BPS
In a helmet with MIPS Brain Protection System the shell and the liner are separated by a low friction layer. When a helmet with MIPS Brain Protection System is subjected to an angled impact, the low friction layer allows the helmet to slide relative to the head. The brain is surrounded by a low-friction cushion of cerebrospinal fluid that protects it by allowing it to slide slightly on impact. MIPS imitates the brain’s way of protecting itself by giving the helmet its own low-friction layer between the outer shell and the liner, which also slides to absorb much of the energy created by an angled blow to the head.
Clinical applications
Pre-operational direct brain functional mapping
CE mark and FDA clearance received
Company
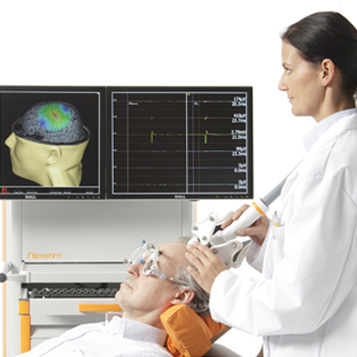
Navigated Brain Stimulation (NBS) System 4
The product is a new navigated brain stimulation device for non-invasive direct cortical mapping in clinical neurosurgery and clinical research into applications of navigated repetitive transcranial magnetic stimulation (rTMS). It combines MRI-guided TMS with EMG measurement and incorporates DICOM export to surgical planning systems, surgical navigators and microscopes. The device is fully self-contained and mobile, does not require a dedicated room or fixed installation, and has a floor footprint of less than 0.6 m2.
More from Nexstim Oy
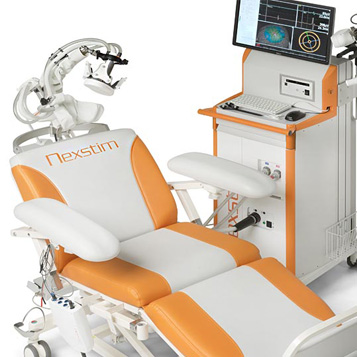
Clinical applications
Stimulation therapy for stroke rehabilitation
CE mark received
2012
Company
NBT® System
NBT® works by using navigated rTMS at a slow frequency in order to help restore the brain´s natural inhibitory balance between its two hemispheres: allowing the injured side of the brain to benefit from subsequent physiotherapy. It is a non-invasive, personalized adjunct therapy to standard physiotherapy.
More from Nexstim Oy

Launched year
2013
Company
Opsumit
Opsumit® (macitentan) is an orally available endothelin receptor antagonist (ERA) that resulted from a tailored drug discovery process with the target of developing an ERA to address efficacy and safety.
More from Actelion Holding Ltd

Launched year
2022
Company
Panic
Panic is a digital therapy program, offering quick and easy access to effective psychological support, tailored to people with a panic disorder or agoraphobia with panic disorder. The course is based on cognitive behavioral therapy and has been clinically proven to reduce the severity of panic symptoms and agoraphobia by an average of 52% six months after completion.
More from HelloBetter

Launched year
2022
Company
Pepaxti
Pepaxti (melphalan flufenamide) is an anticancer peptide-drug conjugate indicated in patients with relapsed or refractory multiple myeloma.
Launch year
2021
Ponvory
Ponvory is an oral sphingosine-1-phosphate receptor modulator indicated for treatment of patients with relapsing multiple sclerosis.

Launched year
2021
Recorlev
Recorlev is a cortisol synthesis inhibitor for the treatment of endogenous hypercortisolemia in adult patients with Cushing’s syndrome for whom surgery is not an option or has not been curative. Endogenous Cushing’s syndrome is a rare but serious and potentially lethal endocrine disease caused by chronic elevated cortisol exposure.
Launched year
2008
Company
Refludan
Refludan® (Lepirudin) is a recombinant hirudin derived from yeast cells and acts as a highly specific direct inhibitor of thrombin. The product is indicated to treat anticoagulation in patients with heparin-induced thrombocytopenia and associated thromboembolic disease.
More from Pharmion Corp.

FDA Approval
2003
Company
Restylane
Restylane is the trade name for a range of injectable fillers with a specific formulation of non-animal sourced hyaluronic acid (HA). Restylane was the first stabilized hyaluronic acid filler on the market and reportedly has been used in over 11 million treatments worldwide. In the United States, Restylane was the first hyaluronic acid filler to be approved by the FDA for cosmetic injection into sub dermal facial tissues.
Indication
Severe aortic stenosis patients who are too ill or frail to have open-heart surgery for aortic valves replacement
CE mark received
2007
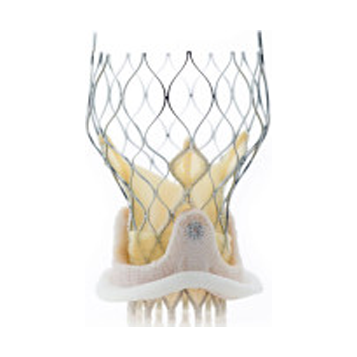
Company
ReValvingTM system
Corevalve has developed the ReValvingTM system for replacing an aortic heart valve by a peripheral, intravascular, percutaneous approach. The device is based on a catheter delivery system containing a self-expanding frame hosting a tissue valve. The product has revolutionized the medical practice moving from open surgery to a percutanous process where the valve together with its frame will be placed in the heart via a peripheral vessel.

Indications
Cardiac arrest; stroke
CE mark received
2011
RhinoChill IntraNasal Cooling System
RhinoChill® IntraNasal Cooling System is a hypothermal device that simultaneously implement conductive cooling, evaporative cooling and convective cooling system to facilitate heat transfer. The product uses a transnasal evaporative catheter to deliver a mist of liquid perfluorocarbon into the nasal cavity. The perfluorocarbon evaporates on contact with the internal nares and base of skull, facilitating rapid and significant heat transfer. It is the first product with mild therapeutic hypothermia to effectively preserve the brain during cerebral ischemia events.
Launched year
2006
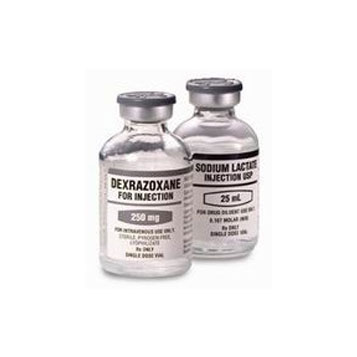
Savene
Savene® (dexrazoxane), developed by TopoTarget (Onxeo), is used for the treatment of anthracycline extravasation, a rare complication to chemotherapy. The product works by inhibiting DNA topoisomerase II, which is the target of anthracycline chemotherapy.

Sleep
Sleep was approved as HelloBetter’s sixth prescribed digital therapy. HelloBetter Sleep is the first prescription digital therapeutic that is approved not just for the treatment of nonorganic insomnia, but also organic insomnia in Germany. In the main study, nearly 80 percent showed a significant reduction in insomnia symptoms after completing the program. The positive effects persisted even six months after the end of the program.
More from HelloBetter
Company
Spacevision, C3, Ulis, Lumis, P.l.u.s, Flex2, UniThread
A series of innovative products which offer key advantages to surgeons and benefits to patients. SpineVision’s current products offer solutions for approximately 90% of spinal pathologies i.e. lumbar degenerative disc diseases, deformities, cervical disorders, trauma and tumors. With its new generation products Flex+2 and LUMIS SpineVision® is the only manufacturer offering a dynamic stabilization system which can be used in a percutaneous approach.

Launched year
2021
Company
Stress and Burnout
The Stress and Burnout course is based on cognitive behavioral therapy and patients learn how to reduce the effects of stress through – among others – psychoeducation, behavioural activation and mindfulness techniques.Eight clinical studies have proven its effectiveness in reducing stress, depressive symptoms, insomnia and symptoms of exhaustion.
More from HelloBetter
Launched year
2021
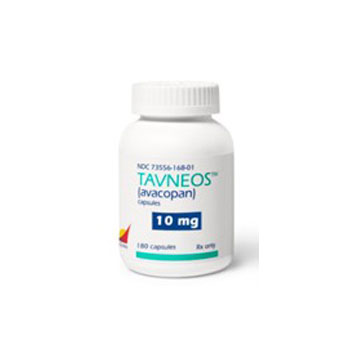
Company
Tavneos
Tavneos (avacopan) is a complement C5 receptor inhibitor indicated for the treatment of patients with Anti-Neutrophil Cytoplasmic Autoantibody (ANCA) vasculitis, a systemic autoimmune disease characterized by destruction of small blood vessels leading to organ damage and failure (typically kidneys), which often is fatal if not treated.
Launched year
1998
Company
Thalomid
Thalomid® (thalidomide) is an immunomodulator used to treat and prevent moderate to severe skin lesions caused by leprosy. The product is also used to treat multiple myeloma in combination with dexamethasone.
More from Pharmion Corp.

Launched year
2001
Company
Tracleer
Being the lead product of Actelion, Tracleer® (bosentan) – an endothelin receptor antagonist – was the first oral treatment approved for PAH.
More from Actelion Holding Ltd
Launched year
2014
Company
Translarna
Translarma (ataluren) is a protein restoration therapy that treats Duchenne muscular dystrophy (DMD). The product was launched in Germany after conditional approval for marketing in the EU states was granted by the European Commission in August 2014. It is the first treatment approved for DMD.
Launched year
2016
Company
Uptravi
Uptravi is used to treat Pulmonary Arterial Hypertension (PAH).
More from Actelion Holding Ltd

Launched year
2022
Company
Vaginismus Plus
The Vaginismus Plus course was developed by experts from science, psychotherapy and psychology, incorporating input from people who suffer from vaginal pain. Patients will learn what could be causing the pain, gain valuable insights into the female body, pleasure and arousal, and learn how to effectively perform vaginal training using dilators with the aim of making vaginal penetration possible by the end of the course. The effectiveness of the course has been examined and confirmed in a clinical trial where 34% of participants were able to have sexual intercourse after completing the course.
More from HelloBetter
Launched year
2010
Company
Veletri
Veletri® (Epoprostenol for Injection) is intravenous prostacyclin. Unlike other epoprostenol formulations approved for PAH, Veletri is stable at room temperature for up to 48 hours when administered immediately upon reconstitution and dilution, making the use of frozen gel packs unnecessary.
More from Actelion Holding Ltd
Launched year
2007
Company
Ventavis
Ventavis® (inhaled iloprost) is an inhaled formulation of iloprost, a synthetic compound that is structurally similar to prostacyclin (PGI2) – a naturally occurring molecule that causes blood vessels to dilate, limits cellular hypertrophy, and inhibits platelet aggregation.
More from Actelion Holding Ltd

Company
VICTUS™ Femtosecond Laser
The VICTUS™ Femtosecond Laser is a versatile platform with cataract, LASIK flap, and therapeutic indications.
Launched year
2008
Company
Vidaza
Vidaza® (azacitidine) is a pyrimidinenucleoside analog of cytidine which induces antineoplastic activity. The product is approved by the FDA and is indicated for the treatment of high-risk myelodysplastic syndrome.
More from Pharmion Corp.
Launched year
2017
Company
Vyzulta
VYZULTA™ (latanoprostene bunod) is a prostaglandin analog indicated for the reduction of intraocular pressure in patients with open-angle glaucoma or ocular hypertension.

Launched year
1999
Company
Wartec
Wartec cream® (podofilox) is an over the counter topical solution formulated to treat genital warts that are found in the external genital region (penis or vulva) or around the anus, caused by human papillomavirus (HPV).

Launched year
2013
Company
Xofigo
Xofigo® (radium Ra 223 dichlorideis) is the first and only approved alpha particle-emitting radiopharmaceutical product for the treatment of castration-resistant prostate cancer with symptomatic bone metastases.
Launched year
2003
Company
Zavesca
Miglustat, the active ingredient of Zavesca®, is an orally available molecule with a large volume of distribution, which was developed for the treatment of type 1 Gaucher disease (GD1).
More from Actelion Holding Ltd
Zephyr Endobronchial Valve
Endobronchial valve (EBV) therapy based on Chartis treatment planning is an effective, minimally invasive method developed by Pulmonx to manage hyperinflation.1 It is intended to predict and achieve reduction of volume in the diseased portion of the lung, without the risks and complications of surgery. EBV therapy involves an assessment of the patient’s hyperinflated lobes for the presence of collateral ventilation, or airflow within or between lobes of the lung, and then treating the patient by placing small, one-way valves in the targeted airways to direct the flow of air out of diseased portions of the lung. A typical procedure involves a Chartis assessment which typically takes about 5 minutes per lobe, and then placing three to four valves in the target lobe, which takes approximately 10 to 30 minutes to complete.
More from Pulmonx Corp.

Company
Zephyr® Valve Delivery System
The Zephyr®Endobronchial Valve is delivered to the target airway using a flexible delivery catheter which is inserted through standard bronchoscopes with a 2.8 mm or larger working channel, and 550 mm or 600 mm working length, depending on the manufacturer. Once positioned in the target bronchus, an actuator button on the catheter retracts the sheath, allowing the Zephyr® valve to expand against the bronchial wall. A typical procedure involves placing approximately three to four valves to isolate the target lobe, and takes approximately 10 to 30 minutes to complete.
More from Pulmonx Corp.
Launched year
2013
Company
Zubsolv
Zubsolv® (buprenorphine and naloxone) sublingual tablet is indicated for treatment of adult opioid dependence. The product should be used as part of a comprehensive treatment plan, which includes counseling and psychosocial support.


